Abstract
Breast cancer is a common but heterogeneous disease characterized by several biologic features, including tumor grade, hormone receptor status, human epidermal growth factor receptor 2 status, and gene expression assays. These biologic and genomic features drive treatment decisions. In the advanced disease setting, inter- and intrapatient tumor heterogeneity is increasingly recognized as a challenge for optimizing treatment. Recent evidence and the recent approval of novel radiopharmaceuticals have increased recognition and acceptance of the potential of molecular imaging as a biomarker to impact and guide management decisions for advanced breast cancer.
Breast cancer represents a broad spectrum of diseases with treatment outcomes varying on the basis of disease stage and inherent tumor biology. Precision medicine aims at treatment customization based on a patient’s specific disease, the disease’s molecular makeup, and the environmental factors in the patient’s life (1). In the late 1970s, tamoxifen, a selective estrogen receptor (ER) modulator that blocks the effects of estrogen, was approved by the U.S. Food and Drug Administration (FDA), becoming one of the first agents in the precision medicine arsenal (2). Numerous other targeted therapies have since been approved. Target identification relies on examining tissue from the biopsy of the primary tumor or a metastatic site.
Molecular imaging is the “visualization, characterization, and measurement of biological processes at the molecular and cellular levels in humans and other living systems (3).” Molecular imaging with radiotracers, by providing functional information, is thereby distinguished from anatomic imaging, which is currently used more often for systemic staging, detecting recurrent disease, and assessing response to therapy in patients with advanced breast cancer.
Recent evidence and the approval of novel radiopharmaceuticals have driven recognition and acceptance of the potential of molecular imaging as a biomarker to guide management decisions for advanced breast cancer. After providing a brief background on breast cancer pathophysiology, this narrative review summarizes the current treatment paradigms and the expanding role of molecular imaging as a precision medicine biomarker for advanced breast cancer.
ANATOMIC AND PROGNOSTIC INDICATORS OF BREAST CANCER
Most breast cancers are carcinomas. The 2 most common histologic subtypes are infiltrating ductal (∼76%) and infiltrating lobular (∼8%) (4). Infiltrating ductal carcinomas typically present as firm masses on physical examination. They invade surrounding tissue in a nonregular pattern, and the malignant cells cause a fibrous reaction in the normal tissue. In contrast, infiltrating lobular carcinoma may be nonmasslike, invading normal tissue in a linear, single-cell–like infiltrative pattern (5). Both histologic subtypes may be detected on screening mammography and receive similar treatment based on clinical stage and molecular subtype. Compared with infiltrating ductal carcinomas, classic infiltrating lobular carcinomas are often of lower grade and larger, with a higher incidence of bilateral involvement at diagnosis, and are more challenging to detect by mammography (6–8).
Hormone receptor expression (ER and progesterone receptor [PR]), human epidermal growth factor receptor 2 (HER2) overexpression or gene amplification, histologic grade, the Ki-67 proliferation marker, and genomic profiling classify breast cancer into distinct clinical subtypes with differing prognoses and treatment paradigms. Gene expression profiling identifies intrinsic subtypes (luminal A, luminal B, basal, and HER2-enriched), which can be approximated by immunohistochemical findings obtained in clinical practice (Table 1) (2,9).
Major Molecular Subtypes of Breast Cancer (9)
BREAST CANCER STAGING
Breast cancer staging follows the American Joint Committee on Cancer staging system, which includes biologic features such as tumor grade; HER2, ER, and PR status; and genomic characteristics (10). Anatomic stage considers primary tumor size, nodal status, and the presence or absence of distant metastases (Tables 2 and 3). Clinical T, N, M, and biomarker information from genomic assays (Oncotype DX [Exact Sciences], MammaPrint [Agendia]) determines pathologic prognostic stage.
Description of T, N, and M Stages for Breast Cancer (10)
TNM Stages for Early, Locally Advanced, and Metastatic Breast Cancer (10)
Advanced breast cancer includes locally advanced breast cancer (LABC), inflammatory breast cancer (IBC), and metastatic breast cancer (MBC). Historically, LABC was defined clinically as those breast cancers deemed inoperable at presentation. LABC includes patients with anatomic stage 3 disease and some with stage 2B (10,11). IBC is clinically distinct, with the diagnosis being based on findings including breast pain, edema, erythema, a rapidly enlarging breast, and a peau d’orange appearance. IBC has a higher likelihood of regional and distant metastases (12). Though IBC technically meets the criteria for LABC, the natural history, treatment paradigms, and outcomes differ from non-IBC (12). MBC, or stage 4 disease, involves organs and lymph nodes outside the locoregional nodal stations. Treatment is generally considered palliative; however, survival for some patients with de novo metastatic HER2-positive or oligometastatic (<5 distant sites) breast cancer may be prolonged (13).
MOLECULAR IMAGING AS A BIOMARKER: KEY TERMINOLOGY
Precision medicine uses biomarkers, defined as a “characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or intervention, including therapeutic interventions” (14). The FDA–National Institutes of Health Biomarker Working Group established the BEST (Biomarkers, EndpointS, and other Tools) Resource to provide a comprehensive glossary of all biomarker types (14). For advanced breast cancer, several biomarkers, including blood, tissue, and imaging measures, play a role in clinical care, and several key terms are important.
A prognostic biomarker correlates with a future event or disease outcome with or without treatment; a key example is hormone receptor status. Patients with ER- or PR-positive tumors survive longer than those with hormone receptor–negative tumors (15). A predictive biomarker determines potential benefit derived from a specific treatment based on the biomarker’s presence or absence. Predictive biomarkers in advanced breast cancer include HER2 overexpression and ER positivity to predict response to HER2-targeted or endocrine therapy, respectively (16,17).
Biomarkers may be both prognostic and predictive (i.e., ER). An integral biomarker directs decision-making in clinical practice or clinical trial settings. An integrated biomarker is included and under investigation in a clinical trial setting but is not used to make decisions (3).
LABC
Treatment
Neoadjuvant systemic therapy is recommended in the setting of LABC to decrease primary tumor size or to make an unresectable primary tumor operable, reduce distant recurrence risk, and inform adjuvant therapy choice based on neoadjuvant treatment response. Achieving a pathologic complete response (pCR, i.e., absence of invasive breast cancer in the breast and axillary nodes) with neoadjuvant therapy reduces recurrence risk, particularly in HER2-positive and triple-negative breast cancer (TNBC) (18).
Breast cancer subtype dictates neoadjuvant therapy selection. HER2-targeted agents (trastuzumab, pertuzumab) are used for HER2-positive breast cancer. Chemotherapy remains the backbone of neoadjuvant and adjuvant treatment for TNBC. Recent studies demonstrated the benefit of adding the immune checkpoint inhibitor pembrolizumab to neoadjuvant chemotherapy for patients with stage 2 or 3 TNBC (19,20). For patients with germline BRCA1, 2 mutations, and high-risk HER2-negative breast cancer, adjuvant olaparib is recommended on the basis of improvements in disease-free and overall survival (21).
For ER-positive, HER2-negative breast cancer, neoadjuvant chemotherapy is associated with a substantially lower pCR rate (18,20). There is emerging interest in a role for immune checkpoint inhibitors for patients with high-risk luminal breast cancers (19). After local therapy, adjuvant endocrine therapy is recommended for hormone receptor–positive breast cancer, with duration varied depending on the clinical risk at presentation and agent used. For premenopausal patients with LABC, ovarian function suppression and aromatase inhibitors are recommended (22). Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors are considered in the adjuvant setting in specific clinical situations, such as high-risk hormone receptor–positive, HER2-negative breast cancer (23).
For all breast cancer subtypes, breast surgery follows neoadjuvant systemic therapy. Multiple factors influence choice of breast conservation versus mastectomy and axillary lymph node dissection versus sentinel lymph node biopsy or targeted axillary dissection. Radiation therapy reduces the risk of local or regional disease recurrence in LABC, even after mastectomy, because of nodal disease involvement at presentation.
Neoadjuvant therapy for LABC is an optimal setting for investigating molecular imaging biomarkers because the pCR endpoint obtained at surgery is a surrogate of survival outcomes (24). The increasing drug armamentarium available in the neoadjuvant setting also provides opportunities to evaluate biologic tumor changes related to the drug’s mechanism of action.
Imaging
Accurate staging of LABC is essential to guide the initial treatment plan, and the primary modalities for initial staging are CT, bone scanning, and 18F-FDG PET/CT. National Comprehensive Cancer Network guidelines endorse 18F-FDG PET/CT for initial staging of at least stage 3 and in select cases of stage 2A or 2B disease in which CT or bone scanning is equivocal or there is a high suspicion of metastatic disease (11). Occult metastases occur in 6%–14% of patients undergoing initial staging 18F-FDG PET/CT, with increasing frequency as stage increases. Up to 30% of patients with stage 3 disease may be upstaged, with similar rates across triple-negative, HER2-positive, and HER2-negative disease (25–27).
Although 18F-FDG PET/CT is increasingly recognized as a single imaging modality for staging LABC (28), its clinical use for this purpose remains variable and even debated, driven mainly by a lack of homogeneous prospective data on how upstaging to stage 4 disease affects clinical outcomes for those otherwise thought to have curable disease. A prospective, randomized trial in patients with stage 2B or 3 invasive ductal carcinomas confirmed more upstaging (n = 43/184, 23.3%) with 18F-FDG PET/CT than with conventional imaging (n = 21/185, 11.3%), leading to less curative-intent treatment in the 18F-FDG PET/CT group. Longer-term data are still needed to determine whether the changed treatment approach affected survival outcomes and whether these results will further standardize 18F-FDG PET/CT use for initial staging of 2B/3 disease. These data do provide evidence that tips the scales to further investigate 18F-FDG PET/CT as an imaging biomarker to explore novel treatment strategies in clinical indications with unmet needs, such as oligometastatic disease, and also demonstrate that randomized imaging trials testing relevant clinical endpoints in specific populations are feasible and of interest (29).
Beyond initial staging, a significant advantage of molecular imaging across many tumor types is visualization of changes indicating response or lack of response before anatomic imaging. Such early changes provide the opportunity for response-adapted therapy. Early during preoperative therapy,18F-FDG PET/CT measures the pharmacodynamic response of breast cancer to predict the likelihood of a pCR at surgery (30,31), mostly studied in HER2-positive disease. In TBCRC026, 83 women with newly diagnosed stage 2 or 3 HER2-positive breast cancer underwent 18F-FDG PET/CT before and 15 d after starting pertuzumab and trastuzumab (31). Most strikingly, patients with less than a 40% decrease in SUVmax at day 15 were unlikely to achieve a pCR at surgery, with a high negative predictive value of 91% (31). A cycle 1, day 15, SUVmax of 3 or less in the primary tumor may also be associated with recurrence-free and overall survival at a 53.7-mo median follow-up (32).
The DIRECT trial (NCT05710328) aims to validate the results of TBCRC026 across several standard neoadjuvant regimens for HER2-positive LABC to subsequently use interim 18F-FDG PET/CT as an integral biomarker to test optimization strategies for patients with HER2-positive disease. PHERGain (NCT03161353) demonstrated the feasibility of this response-adapted approach (30). In treatment arm B, early 18F-FDG PET/CT adds chemotherapy to trastuzumab and pertuzumab if more than a 40% decline in SUVmax is not observed. The results of these important trials are eagerly awaited.
Studies of early 18F-FDG PET/CT for predicting pCR in TNBC cancer have yielded mixed results and been limited by small sample sizes and various treatment regimens (33–36). Larger well-designed studies are needed, but this represents a clinical need for which treatment optimization would be highly beneficial.
Another advantage of PET imaging is the ability to perform dynamic imaging and derive tumor kinetics. In 75 patients with LABC who underwent 18F-FDG PET at baseline and midway through neoadjuvant chemotherapy and after adjusting for ER status and axillary stage, models including kinetic parameters (K1 and inhibition constant [flux]) for predicting pCR were more robust than SUV (area under the receiver-operating-characteristic curve, 0.97 vs. 0.84; P = 0.005). Further changes in K1, but not SUV, independently prognosticated for disease-free and overall survival (37). Practically, deriving kinetic PET parameters is more complex than deriving static parameters but is feasible. Kazerouni et al. evaluated changes in dynamic 18F-FDG PET and dynamic contrast-enhanced MRI prospectively in 35 patients with LABC (38). They found that mid-treatment changes in both 18F-FDG PET and dynamic contrast-enhanced MRI measures were predictive of pathologic response by residual cancer burden and recurrence-free survival after neoadjuvant chemotherapy. The 2 modalities offer complementary measures of metabolism and perfusion, and greater reductions in metabolism–perfusion mismatch were associated with improved recurrence-free survival. These noninvasive imaging-based markers could help guide treatment decisions and facilitate more personalized therapies for optimal patient outcomes.
18F-3′-deoxy-3′-fluorothymidine (18F-FLT) images tumor proliferation, correlates with Ki-67 (39), and has generated interest as a biomarker for predicting LABC response to preoperative chemotherapy. Crippa et al. found that changes in tumor 18F-FLT SUVmax could separate responders with residual cancer burden 0 + 1 from those with residual cancer burden 2 + 3 and proposed a predictive score (40). The prospective phase 2 ACRIN 6688 study showed that changes in 18F-FLT uptake could predict pCR after 1 cycle or at the completion of neoadjuvant therapy, but with a higher area under the curve (0.83 vs. 0.68) at the later time point (39). Additional smaller studies also demonstrated potential for serial 18F-FLT as a predictive imaging biomarker (39,41–43).
Despite its promise, several factors limit 18F-FLT’s clinical applicability. 18F-FLT is not widely available, nor is it FDA-approved. High uptake in the bone marrow and liver limit evaluation of these organs and use for initial staging. 18F-FLT may predict pCR better after neoadjuvant therapy, whereas 18F-FDG PET/CT may be predictive within 2 wk of starting neoadjuvant therapy, sparing exposure to ineffective therapy. Consequently, at present, 18F-FLT PET is unlikely to supplant 18F-FDG as an imaging biomarker for predicting pCR.
Furthermore, as more targeted drugs become available in the neoadjuvant setting for LABC, it will be essential to match the therapeutic drug mechanism of action with the radiotracer mechanism of uptake and even downstream processes to identify and optimize the use of molecular imaging biomarkers.
MBC
Treatment
MBC is generally not considered curable, although patients with HER2-positive breast cancer may experience long disease-free periods because of highly efficacious therapies (44). If metastatic disease presents at diagnosis, surgical resection and radiation therapy are not typically options but may become appropriate if tumor burden affects the quality of life (45). Treatment recommendations are based on tumor biology, previous treatments, disease burden, patient’s performance status, preferences, and comorbidities (46). The acquisition of metastatic tumor tissue and evaluation of genetic makeup for actionable mutations (PIK3CA, ESR1), tumor mutational burden, and microsatellite stability are recommended to inform systemic therapy. Medical genetics counseling and germline testing are also recommended for all patients with MBC because of the efficacy of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in patients harboring germline BRCA1, BRCA2, and PALB2 mutations. However, the treatment goal is often more individualized and centers around symptomatic management after providing systemic therapy (47).
For hormone receptor–positive tumors, first-line systemic therapy usually consists of endocrine therapy with a CDK4/6 inhibitor. Endocrine therapies include selective ER modulators (i.e., tamoxifen), aromatase inhibitors, and selective ER degraders (i.e., fulvestrant and elacestrant) (47). Prolongation of overall survival has been demonstrated with targeted treatments such as CDK4/6 inhibitors (ribociclib, abemaciclib), and prolongation of progression-free survival (PFS) has been demonstrated with CDK4/6 inhibitors, mammalian-target-of-rapamycin inhibitors (i.e., everolimus), and alpelisib (phosphatidylinositol-3′-kinase inhibitor) (47). Resistance to first-line therapy is common. For patients with tumors harboring ESR1 mutations, elacestrant demonstrated improved PFS versus standard-of-care endocrine therapy and received FDA approval in 2023 (48). Once endocrine resistance has been established, systemic therapy options for patients with ER-positive, HER2-negative breast cancer include sequential chemotherapy and antibody–drug conjugates (49,50).
For HER2-positive MBC, first-line standard treatment is trastuzumab and pertuzumab (anti-HER2 monoclonal antibodies) and taxane chemotherapy (47). The second-line standard is presently a HER2-targeted antibody–drug conjugate (trastuzumab emtansine). However, many other highly effective HER2-targeted agents are available in the advanced disease setting. Most recently, in the DESTINY-Breast03 trial, trastuzumab deruxtecan demonstrated a significant improvement in overall survival versus trastuzumab emtansine (51).
For TNBC, chemotherapy is the treatment mainstay. For patients with PDL1-positive tumors (assessed by a combined positive score ≥ 10%), pembrolizumab plus chemotherapy improved PFS in the KEYNOTE 355 trial (52). Recently developed highly potent antibody–drug conjugates offer additional therapy options for patients with metastatic TNBC. Sacituzumab govitecan is a monoclonal antibody against Trop2 conjugated via a cleavable linker to SN-38, the active metabolite of irinotecan. The ASCENT trial reported improved PFS and overall survival for sacituzumab govitecan versus the physician’s choice of chemotherapy for advanced TNBC and led to FDA approval in 2021 (53). For patients with germline BRCA-associated TNBC, incorporation of platinum is associated with higher objective response rates (54) and PARP inhibitors are recommended on the basis of the results of the OlympiAD and EMBRCA trials (55,56).
For patients with osseous metastases, regardless of breast cancer subtype, bone-modifying drugs such as bisphosphonates or denosumab are recommended to reduce the risk of skeleton-related complications (hypercalcemia of malignancy, pathologic fractures, or need for radiation) (47).
Imaging
The current radiopharmaceuticals approved for assessing MBC are 18F-FDG, 18F-NaF, and 18F-fluoroestradiol (18F-FES). 18F-FDG PET/CT better detects recurrent disease and lytic bone metastases than conventional imaging (i.e., CT, MRI, and bone scanning) (57,58). 18F-FDG PET/CT is also a valuable biomarker for response and outcome, particularly for patients with bone-dominant or bone-only MBC, who are often excluded from drug trials because of lack of measurable disease by RECIST 1.1. In 28 women with bone-dominant or bone-only MBC, Peterson et al. demonstrated that changes on 18F-FDG PET/CT after 4 mo of standard-of-care treatment predicted time to skeletal-related event and time to progression but not overall survival using modified PERCIST (59). Serial 18F-NaF PET/CT did not predict time to skeletal-related event and time to progression but did predict overall survival. Makhlin et al. recently reported longer, albeit nonsignificant, PFS, overall survival, and time to skeletal-related event in 23 women with ER-positive bone-dominant or bone-only MBC (60). The lack of significance could be related to the small sample size. The FEATURE/EA1183 clinical trial (NCT04316117) is under way to validate these findings. If validated, 18F-FDG PET/CT may serve as an imaging biomarker in routine practice and clinical trials for this group of patients.
Given that endocrine therapy is the backbone of treatment for ER-positive disease, the 2020 FDA approval of 18F-FES has opened a potential door to advance precision medicine for patients with ER-positive MBC. 18F-FES selectively binds ER, and in contrast to tissue and blood biomarkers, 18F-FES PET/CT surveys the whole body to assess tumor burden heterogeneity, functional expression of the target, and ligand binding. This is relevant because over the disease course, hormone receptor status may change 30%–41% of the time, and loss of initial ER positivity increases risk of death compared with stable ER status (61).
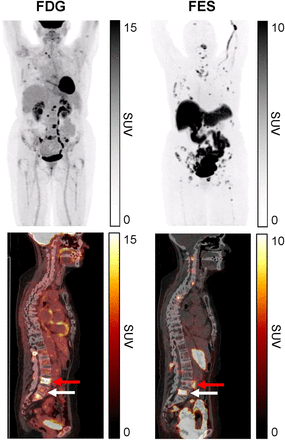
18F-FES PET/CT can help clarify ER tumor status and distinguish the origin of the metastasis in the setting of multiple primary breast malignancies (62). 18F-FES PET/CT detects infiltrating lobular cancer metastases with higher sensitivity than 18F-FDG (Fig. 1), particularly osseous metastases, though larger trials are required (63). In 16 women with ER-positive MBC undergoing 18F-FDG PET and 18F-FES PET before rintodestrant therapy 18F-FES PET was prognostic, with a trend for longer PFS with higher 18F-FDG and 18F-FES uptake (64). Baseline tumor 18F-FES uptake has also been suggested to predict responsiveness to endocrine therapy in those with ER-positive disease (65). EAI142 (NCT02398773) investigated the negative predictive value of 18F-FES PET/CT for clinical benefit at 6 mo of endocrine treatment, and the results are awaited to help design future clinical trials. For predicting endocrine therapy response, 18F-FES PET/CT primarily characterizes tumor for functional target, and correlation with concurrent 18F-FDG PET/CT is likely important to quantify tumor heterogeneity, that is, burden of ER-positive and ER-negative metastases (16).
66-y-old woman with de novo metastatic, ER-positive lobular breast cancer. 18F-FES PET/CT shows more lesions than 18F-FDG PET/CT. ER tumor heterogeneity is also demonstrated with both 18F-FDG–negative, 18F-FES–positive lesions (white arrows) and 18F-FDG–positive, 18F-FES–negative lesions (red arrows).
FUTURE DIRECTIONS OF MOLECULAR IMAGING AND THERAPY IN LABC
Other radiotracers have been or are being explored for molecular imaging of LABC. A full review of all of these is beyond this article’s scope. In this section, several promising radiotracers that image pathways already targeted for treatment in LABC are reviewed.
As previously discussed, PARP inhibitors are efficacious in patients with metastatic HER2-negative breast cancer with germline BRCA1 and BRCA2 mutations (28). Several PARP-targeting agents have been radiolabeled (66,67). In early-phase clinical trials, 18F-fluorthanatrace uptake in tumors was variable but correlated with PARP-1 expression. In 4 patients with stage 3 or 4 breast cancer, 3 with increased 18F-fluorthanatrace uptake at baseline had a decline in uptake after PARP inhibitor therapy, with a partial response or stable disease. The fourth patient had no 18F-fluorthanatrace uptake at baseline and had subsequent disease progression. These, and other (68), early data on radiolabeled PARP inhibitors suggest a potential future role as a pharmacodynamic or predictive imaging biomarker for those being considered for PARP inhibitor therapy.
The HER2-targeted therapies trastuzumab and pertuzumab have both been radiolabeled for noninvasively imaging HER2 expression. In a study of 24 women with HER2-negative primary breast cancer, 6 had 89Zr-pertuzumab uptake in metastases: 3 HER2-positive on biopsy, 2 negative, and 1 inconclusive (69). In a single-institution study of 50 patients (34 with HER2-positive disease) and using an SUVmax cutoff of 3.2, 89Zr-trastuzumab PET/CT correctly characterized HER2 status with a sensitivity of 76%, specificity of 62%, positive predictive value of 83%, and negative predictive value of 50%. Twenty percent of patients with multiple lesions had variable 89Zr-trastuzumab uptake (70). Like ER, the global in vivo assessment of tumor heterogeneity overcomes the limitation of assessing HER2 status from a single biopsy site or when biopsy is not feasible (69–71). HER2-targeted PET could help identify those who may have otherwise been thought not likely to benefit from HER2-targeted therapy, particularly with emergence of the HER2-low category, which benefits from some of the newer HER2-targeted drugs, likely trastuzumab deruxtecan (49). Both trastuzumab and pertuzumab have also been radiolabeled with therapeutic radioisotopes for theranostic application, but work in this domain is early (72).
Endocrine therapy for advanced breast cancer currently targets the ER. Over time, resistance to endocrine therapy develops. PR imaging with 18F-fluorofuranylnorprogesterone has been evaluated, and in vitro studies demonstrated that changes in PR expression could provide insight into the development of resistance to endocrine therapy (73).
Fibroblast activation protein has emerged as a new diagnostic and therapeutic target across a variety of cancer types (74), with multiple radiolabeled fibroblast activation protein inhibitors (FAPIs) under investigation. Stromal cancer-associated fibroblasts and tumor-associated macrophages express fibroblast activation protein in all breast cancer subtypes (75). Early studies of FAPI PET/CT in breast cancer were generally small, used different FAPI radiotracers, and included heterogeneous subtypes of breast cancer but consistently demonstrated increased FAPI uptake and tumor-to-background ratios in primary breast cancer, lymph node metastases, and bone metastases compared with 18F-FDG (66,76). FAPI PET/CT appears advantageous for detecting smaller lesions and those breast cancers with low-level 18F-FDG uptake (77).
Backhaus et al. evaluated the use of 68Ga-FAPI-46 PET/MRI for predicting pCR after neoadjuvant therapy in 13 women with mixed subtypes of invasive breast cancer (78). After neoadjuvant therapy, those with pCR had a lower FAPI-to-background ratio than those with no pCR. A limited number of patients with MBC have been treated with 177Lu- or 90Y-labeled FAPI. Adverse events were manageable, with several instances of stable disease or partial response reported (79). Fibroblast activation protein–targeting breast cancer for imaging and therapy seems feasible. Still, the data are too early to draw conclusions about the future use of precision medicine in specific subtypes of breast cancer.
CONCLUSION
Given the heterogeneity of advanced breast cancer, precision medicine and targeting of different biomarkers have proven highly beneficial in treatment, with many other potential therapeutic targets currently under investigation. Several approved radiotracers are also now available or under investigation. Molecular imaging is uniquely positioned to aid in treatment planning by detecting disease, determining disease extent, and characterizing biomarker status in vivo and across the entire disease burden. As medical oncology and molecular imaging continue to evolve, it will remain essential to match processes based on biologic mechanisms for treatment and imaging to take advantage of the full potential of precision medicine. Finally, some of these imaging biomarkers may not need clinical implementation. They could also be helpful as pharmacodynamic markers to determine optimal dosing for drug development or to study mechanisms of action or resistance.
ACKNOWLEDGMENTS
We thank Dr. Lanell Peterson for assistance with Figure 1.
Footnotes
Learning Objectives: On successful completion of this activity, participants should be able to (1) define prognostic and predictive imaging biomarkers and name at least 1 prognostic and predictive imaging biomarker for advanced breast cancer; (2) list the current roles of 18F-FDG PET/CT imaging in advanced breast cancer; and (3) list 3 non–18F-FDG radiotracers that may serve as imaging biomarkers in advanced breast cancer.
Financial Disclosure: Dr. Jacene is a consultant for Blue Earth Diagnostics and Spectrum Dynamics Ltd.; receives research support from Blue Earth Diagnostics, Inc. (to the institute); received speaker honoraria from ITM and Monrol; and receives royalties from Cambridge University Press. Dr. Specht receives research support from Lyell Immunopharma, Merck, Cascadian Therapeutics, Pfizer, Seattle Genetics, Genentech, Xencor, Abbvie, Inc., Celcuity, Inc., Novartis, Myriad Pharmaceuticals, AstraZeneca, and Carisma Therapeutics; is a consultant for GE Healthcare, Sensei Biotherapeutics, Lyell Immunopharma, A2 Biotherapeutics, and Volastra; is on the advisory board for Daiichi Sankyo, GlaxoSmithKline, GE Healthcare, Sensei Biotherapeutics, and Lyell Immunopharma; and is an investigative meeting participant for A2 Biotherapeutics. The authors of this article have indicated no other relevant relationships that could be perceived as a real or apparent conflict of interest.
CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, SAM, and other credit types, participants can access this activity through the SNMMI website (http://www.snmmilearningcenter.org) through March 2027.
Published online Feb. 1, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.
- 35.
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- Received for publication September 27, 2023.
- Accepted for publication January 5, 2024.