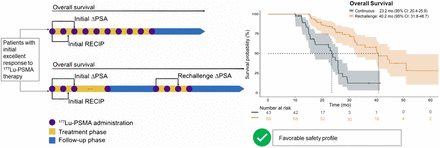
Visual Abstract
Abstract
Prospective results have demonstrated favorable safety and efficacy of [177Lu]Lu-PSMA radiopharmaceutical therapy for up to 6 cycles in men with metastatic castration-resistant prostate cancer. However, no systematic data are available outlining the feasibility of extended therapy beyond 6 cycles. We aim to evaluate the safety and efficacy of extended [177Lu]Lu-PSMA radiopharmaceutical therapy in patients who have received more than 6 cycles. Methods: In total, 111 patients were included in this multicenter retrospective analysis. Based on individual decisions, patients underwent uninterrupted continuation of therapy (continuous treatment) or reexposure after a therapy break (rechallenge treatment) between 2014 and 2023. Overall survival, 50% prostate-specific antigen (PSA) decline (measured 8–12 wk after treatment initiation or rechallenge), PSMA PET response, and grades per Common Terminology Criteria for Adverse Events were assessed. χ2 tests, multivariable Cox regression analysis, and log-rank tests were applied for statistical analyses. Results: Patients received extended treatment with [177Lu]Lu-PSMA, either as a continuous treatment (43/111, 38.7%) or as a rechallenge (68/111, 61.3%) treatment, with median cumulative doses of 57.4 or 60.8 GBq, respectively. Overall survival from the initiation of [177Lu]Lu-PSMA was 31.3, 23.2, and 40.2 mo for the entire cohort, the continuous treatment group, and the rechallenge treatment group, respectively. The initial 50% PSA decline was significantly higher in the retreated group than in the continuous group (57/63 [90.4%] vs. 26/42 [61.9%]; P = 0.006). A 50% PSA decline was observed in 23 of 62 patients (37.1%) after the first rechallenge. The rate of grades 3–4 toxicity was comparable between continuous and rechallenge treatments (anemia, 7/43 [16.3%] vs. 13/68 [19.1%)], P = 0.6; leukocytopenia, 1/43 [2.3%] vs. 2/67 [3.0%], P = 0.3; thrombocytopenia, 3/43 [7.0%] vs. 3/68 [4.4%], P = 0.3; renal, 2/43 [4.7%] vs. 5/68 [7.4%], P = 0.2). Conclusion: Extended therapy with [177Lu]Lu-PSMA is safe and has not been associated with increased grades 3–4 toxicity. Patient candidates for extended treatment experienced a favorable median survival of 31.3 mo from the first administration. Response under [177Lu]Lu-PSMA rechallenge demonstrated preserved efficacy of [177Lu]Lu-PSMA after a treatment break.
Prostate-specific membrane antigen (PSMA)–targeted radiopharmaceutical therapy (RPT) is an approved option for patients with metastatic castration-resistant prostate cancer (mCRPC). The VISION trial demonstrated that [177Lu]Lu-PSMA significantly prolonged overall survival (OS) compared with the control arm (1). The VISION trial allowed up to 6 cycles of 7.4 GBq of [177Lu]Lu-PSMA-617. This was partly based on a German multicenter retrospective study that reported early clinical experience (2).
In the VISION trial, approximately one third of patients in the [177Lu]Lu-PSMA arm did not experience biochemical response, which was defined as a greater than 50% decrease in prostate-specific antigen (PSA) levels (1). In addition, patients who responded to treatment demonstrated highly variable depth or duration of response. It may be beneficial to extend the use of [177Lu]Lu-PSMA beyond 6 cycles, and this approach is currently being applied in clinical practice. However, systematic data on safety and the antitumor effect of [177Lu]Lu-PSMA RPT beyond 6 cycles are scarce (1,3,4).
Treatment extension beyond 6 cycles may be conducted as an uninterrupted continuation of treatment (continuous treatment) or reexposure after a therapy break (rechallenge treatment). Rechallenge treatment is usually conducted following biochemical and imaging responses after initial cycles and disease progression after treatment cessation. Rechallenge treatment in these good responders could potentially delay the risk of [177Lu]Lu-PSMA–related side effects. However, it is unclear whether the initial treatment effect is preserved after a therapy break. Thus, the aim of this retrospective analysis was to evaluate the safety and efficacy of extended [177Lu]Lu-PSMA therapy (i.e., beyond 6 cycles) and to investigate potential differences in safety and efficacy of continuous and rechallenge treatments.
MATERIALS AND METHODS
Patient Cohort and [177Lu]Lu-PSMA RPT
The data from patients who underwent more than 6 cycles of [177Lu]Lu-PSMA therapy between December 2014 and March 2023 were retrospectively extracted from the databases of the University Hospitals in Augsburg, Essen, Münster, and Munich (Technical University). All patients signed an informed consent form and were treated under the conditions of the Declaration of Helsinki article 37 (unproven interventions in clinical practice). The multicenter retrospective analysis was approved by the ethics committee in Essen (19-8570-BO and 2021-576-f-S), and the retrospective analysis was approved by local ethics committees (Augsburg, 2020-40 and 23-0847; Münster, 2016-585-f-S; and Munich, 115/18S). [177Lu]Lu-PSMA was prepared according to good manufacturing practices and the German Medicinal Products Act (AMG §13 2b) at all respective centers. Either [177Lu]Lu-PSMA-617 or [177Lu]Lu-PSMA I&T was administered as summarized in Table 1.
Patient Characteristics (n = 111)
European Association of Nuclear Medicine guidelines were followed for patient selection for [177Lu]Lu-PSMA therapy, radionuclide preparation, therapy administration, radiation protection, and follow-up monitoring (5,6). The treatment decision was made by an interdisciplinary tumor committee. Patients had to have received androgen deprivation therapy, androgen receptor signaling inhibitors, and at least 1 line of chemotherapy in the absence of contraindications. The degree of PSMA expression was determined by PSMA PET before the initiation of treatment. PSMA PET–based eligibility was based on VISION trial criteria, that is, higher lesion uptake than in the liver and the absence of PSMA-negative lesions. Patients with macroscopic residual disease, that is, stable disease or with a partial response, underwent continued treatment beyond 6 cycles (continuous treatment). Patients showing excellent biochemical and imaging responses by judgment of the treating physician were offered a therapy break after 4–6 cycles and were reexposed to [177Lu]Lu-PSMA RPT (rechallenge treatment). Eligibility and choice of treatment protocol were decided by an interdisciplinary tumor committee. A therapy break was defined as a period of at least 4 mo without [177Lu]Lu-PSMA administration, which was chosen as twice the maximum treatment interval of 8 wk and thus prevented patient logistic-induced breaks from qualifying as rechallenge regimens.
Therapy Response
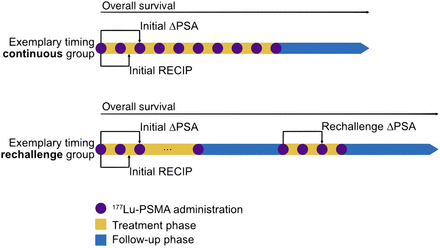
Biochemical response to therapy was defined as a PSA decline of at least 50% 8–12 wk after treatment initiation (initial change in PSA). For patients who underwent rechallenge treatment, the biochemical response at rechallenge was calculated using the first cycle of the rechallenge as a baseline (rechallenge change in PSA). Interim PSMA PET (after 2 cycles of initial [177Lu]Lu-PSMA therapy) was interpreted using visual assessment following Response Evaluation Criteria in PSMA PET/CT (7). Figure 1 shows the assessment of the response to treatment.
Patients received extended administration of [177Lu]Lu-PSMA (>6 cycles), either continuously (continuous treatment) or as reexposure after therapy pause (rechallenge treatment). PSA response was measured at 8–12 wk after treatment initiation and start of rechallenge. Imaging response by Response Evaluation Criteria in PSMA PET/CT (RECIP) was assessed after 2 cycles. ΔPSA = change in PSA.
Adverse Events
Serum parameters, such as hemoglobin, leukocyte, and thrombocyte counts, and kidney and liver function values were recorded during each treatment cycle and, where possible, during 3 mo of follow-up. Adverse events were assessed according to Common Terminology Criteria for Adverse Events version 5.0 for each category.
OS
OS was calculated from the initial [177Lu]Lu-PSMA treatment until loss of follow-up or death.
Statistical Analysis
The R language (The R Project for Statistical Computing) was used for statistical analyses and graphical representation (Kaplan–Meier curve, box, and swimmer plots). Normal distribution was assessed by the Kolmogorov–Smirnov test. Descriptive data were given as mean and SD for normally distributed parameters, as median and interquartile range for skewed parameters, or as number and percentage. Cox regression analysis and log-rank tests were used for censored data, and the χ2 exact test was used for categoric data. A P value of less than 0.05 was considered statistically significant. CIs represent the 95% range.
RESULTS
Patient Characteristics and [177Lu]Lu-PSMA Therapy Regimes
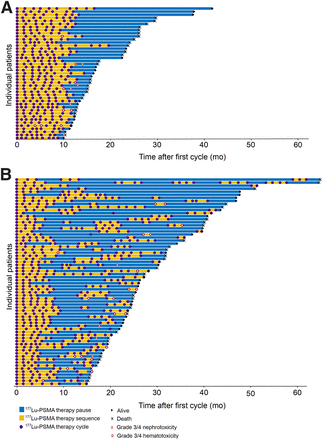
In total, 111 patients were included. Of those patients, 43 (38.7%) received continuous treatment whereas 68 (71.3%) underwent rechallenge treatment (Fig. 2). Median cumulative activity per patient in the continuous and rechallenge treatments was 57.4 GBq (range, 51.6–59.5 GBq) versus 60.8 GBq (range, 54.9–73.1 GBq), respectively, and the median interval between therapy cycles in each sequence of continuous and rechallenge treatments was 1.4 mo (range, 1.4–1.8 mo) versus 1.5 mo (range, 1.4–1.9 mo), respectively. The median interval between the end of the initial [177Lu]Lu-PSMA RPT and initiation of rechallenge treatment was 7.2 mo (range, 5.4–11.5 mo). Of the patients who received continuous therapy, 24 of 43 (55.8%) received therapy once or twice within an interval of 10–16 wk; otherwise, therapy occurred within an interval of 8–10 wk. For the continuous and rechallenge groups, respectively, the median number of treatment cycles was 8 versus 9, and the median dose per cycle was 7.2 versus 6.8 GBq. In the rechallenge group, 80.1% of patients received 1 rechallenge treatment, whereas the remainder received 2–4 additional rechallenge treatments: 10 of 68 (14.7%) received 2 additional treatments, 2 of 68 (2.9%) received 3 additional treatments, and 1 of 68 (1.5%) received 4 additional treatments. Patient characteristics and [177Lu]Lu-PSMA treatment regimes are presented in Tables 1 and 2.
Swimmer plots depicting individual treatment sequence, outcome, and toxicity. Data are presented separately for continuous (A) and rechallenge treatment (B).
[177Lu]Lu-PSMA RPT Regimen
Safety
Higher-grade adverse events according to Common Terminology Criteria for Adverse Events version 5.0 are presented in Table 3 and are illustrated in Figure 3. Rates of grades 3–4 hematotoxicities were not significantly different between the continuous and rechallenge groups for anemia (16.3% vs. 19.1%; P = 0.6), leukocytopenia (2.3% vs. 3.0%; P = 0.3), and thrombocytopenia (7.0% vs. 4.4%; P = 0.3).
Incidence of Grades 3–4 Adverse Events Stratified by Continuous vs. Rechallenge Treatment
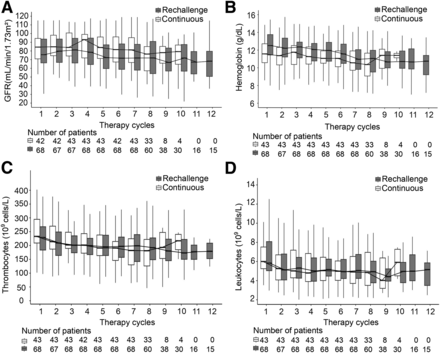
Median serum values for GFR (A), hemoglobin (B), thrombocytes (C), and leukocytes (D) per cycle from patients receiving continuous or rechallenge [177Lu]Lu-PSMA. Data are only shown for first 12 cycles.
Hematologic parameters in the last treatment cycle were significantly lower than those in the first cycle for both the continuous and rechallenge groups (Table 4). In the overall cohort (20 total events), the frequency of grades 3 and 4 anemia was higher in the extended treatment sequence (cycle 7 and beyond) than in the initial treatment sequence (cycles 1–6) (12/20 [60%] vs. 8/20 [40%]). Grade 4 thrombocytopenia occurred in 1 patient during the rechallenge therapy cycles.
Median of Laboratory Parameters During First Therapy vs. Last Therapy Cycle in Both Treatment Groups
Few occurrences of grades 3–4 renal toxicity (7/111 [6.3%]) were observed in the overall cohort, with most occurring in the extended treatment sequence (cycle 7 and beyond) (4/7 [57.1%]). The rate of renal toxicity was not significantly different between the continuous and rechallenge groups (2/43 [4.7%] vs. 5/68 [7.4%]; P = 0.2). Median glomerular filtration rates (GFRs) were significantly lower in the last treatment cycle than in the first in both continuous and rechallenge groups (Table 4).
Any grade of xerostomia was detected in 58 of 111 patients in the overall cohort (52%, n = 29 for the continuous group and n = 29 for the rechallenge group) with most occurring in the initial 6 cycles rather than in the extended treatment sequence (40/58 [69%] vs. 18/58 [31%]). Any grade of dry eyes was detected in 13 of 111 patients in the overall cohort (12%, n = 5 for the continuous group and n = 8 for the rechallenge group), with most occurring in the first 6 cycles rather than in the extended treatment sequence (11/13 [85%] vs. 2/13 [15%]).
Administered activity was reduced to a range of 3 and 6 GBq in 17 patients (5.3%) because of a decrease in GFR (35.3%) and hematotoxicity (64.7%), respectively.
[177Lu]Lu-PSMA RPT was discontinued in 15 patients (15/111 [13.5%]) because of decreased GFR (7/15 [46.7%]) or hematotoxicity (8/15 [53.3%]) (Table 3). In the continuous group, therapy was discontinued in 3 patients because of a decrease in GFR and in 2 patients because of thrombocytopenia. In the rechallenge group, therapy was discontinued in 4 patients because of a decrease in GFR, in 3 patients because of thrombocytopenia, in 1 patient because of bicytopenia, in 1 patient because of pancytopenia, and in 1 patient because of anemia.
Interestingly, patients who had not previously received docetaxel or cabazitaxel had a tendency toward higher rates of adverse events, resulting in dose reduction, than did those who received one or both treatments (7/24 [29.2%] vs. 6/54 [11.1%] vs. 4/33 [12.1%]; P = 0.1).
Biochemical Response
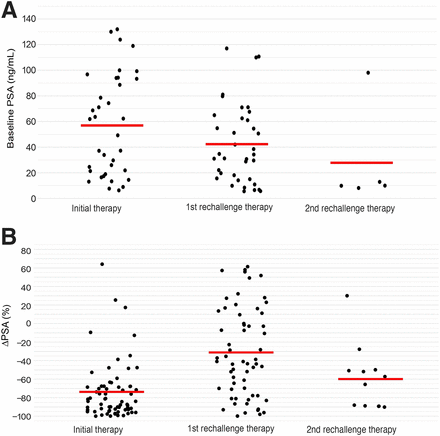
The rate of the initial 50% PSA decline was significantly higher in the rechallenge group than in the continuous group (57/63 [90.4%] vs. 26/42 [61.9%]; P = 0.006; Table 5). In the continuous group, the rate of 50% PSA decline after the first 6 cycles was 36 of 42 (85.7%), whereas the rate of 50% PSA decline between the sixth cycle and the last cycle was 7 of 43 (16.3%). In the rechallenge group, the rate of 50% PSA decline was 23 of 62 (37.1%) after the first rechallenge treatment and 9 of 12 (75.0%) after the second rechallenge treatment. Figure 4 shows individual PSA levels and the 50% PSA decline during the initial treatment and additional rechallenge treatments.
Response to [177Lu]Lu-PSMA RPT Stratified for Continuous vs. Rechallenge Treatment
Individual initial PSA values and PSA decline for patients receiving rechallenge treatment. Baseline PSA values are given before treatment initiation and before rechallenge treatment (A). Initial and subsequent PSA decline are presented after treatment initiation and rechallenge treatments (B). ΔPSA = change in PSA.
Imaging Response
Interim PSMA PET was available in 97 of 111 patients (87.3%) (38/43 in the continuous group and 59/68 in the rechallenge group). In the continuous versus rechallenge groups, partial response by PSMA PET/CT was observed in 10 of 38 patients (26.3%) versus 33 of 59 patients (55.9%), and stable disease was observed in 26 of 38 patients (68.4%) versus 25 of 59 patients (42.4%). The initial partial response rate was significantly lower in the continuous group than in the rechallenge group (26.3% vs. 55.9%, P = 0.004). Details on the PSMA PET response are shown in Table 5.
OS
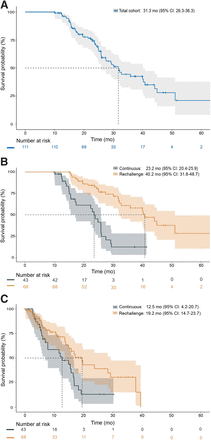
Patient status was followed for a median of 33.9 mo (95% CI, 25.0–42.7 mo) after initiation of [177Lu]Lu-PSMA RPT. A total of 54 patients (51.4%) died, and median OS was 31.3 mo (95% CI, 26.3–36.3 mo) for the entire cohort (Fig. 5A). Figure 5B shows that OS from the beginning of initial [177Lu]Lu-PSMA therapy was significantly shorter for continuously treated patients than for rechallenge patients (23.2 mo [95% CI, 20.4–25.9 mo] vs. 40.2 mo [95% CI, 31.8–48.7 mo]; P < 0.001).
Kaplan–Meier curves for OS from first [177Lu]Lu-PSMA application for entire cohort (A) and separately for continuous and rechallenge treatments (B and C).
DISCUSSION
In this retrospective analysis, we assessed the safety and efficacy of [177Lu]Lu-PSMA therapy beyond 6 cycles. After 6 cycles of standard [177Lu]Lu-PSMA treatment, patients in our cohort either received continuous [177Lu]Lu-PSMA treatment for residual disease or were treated again after a pause because of a good initial response. Extended treatment with [177Lu]Lu-PSMA was well tolerated, with few grades 3–4 adverse events, with the most frequent being anemia after 6 cycles. Overall, extended [177Lu]Lu-PSMA RPT achieved an OS of 31.3 mo from the first administration.
In our study, extended treatment with [177Lu]Lu-PSMA was well tolerated. Grade 3 or 4 anemia was observed in 18% (20/111) of patients, mainly during additional cycles. Grade 3 or 4 thrombocytopenia was observed in only 5% of patients, and grade 3 neutropenia was found in only 0.1% of patients. In line with our results, Mader et al. reported grades 3–4 anemia in 15% of patients receiving extended [177Lu]Lu-PSMA therapy and reversible thrombocytopenia in only 1 patient (4%) (8). In contrast to our cohort, mCRPC patients who received cabazitaxel as second-line chemotherapy had high rates of grades 3–4 neutropenia (82%), and a considerable fraction had febrile neutropenia (8%) (9). However, the rate of grades 3–4 anemia and thrombocytopenia was similar to that found in our study (11% and 4%, respectively) (9). In the TheraP study, the cabazitaxel group had a higher rate of any grade 3 or 4 adverse event overall than did the [177Lu]Lu-PSMA group (53% vs. 33%) (10). The data indicate that initial and extended [177Lu]Lu-PSMA therapy may be tolerated better than cabazitaxel in mCRPC patients after docetaxel chemotherapy.
Our population demonstrated a low rate of grades 3–4 events concerning renal function assessed by the GFR (7/111, 6.3%). In line with our results, only 1 patient (4%) who received 12 cycles of [177Lu]Lu-PSMA treatment experienced grade 3 nephrotoxicity in the Mader et al. study (8). Furthermore, Mader et al. were able to demonstrate that the critical threshold for a renal absorbed dose of 40 Gy was not significantly associated with a reduction in GFR (8). Our results underline favorable renal safety with a low risk of induced renal failure. However, Schäfer et al. reported 3 cases in which patients who received more than 6 cycles of [177Lu]Lu-PSMA therapy showed nephropathy with severe chronic kidney disease that was likely induced by diffuse subacute renal thrombotic microangiopathy as well as acute tubular injury (11). In addition, most recently, Steinhelfer et al. reported that a considerable proportion (45%) of patients may experience moderate to severe decreases in estimated GFR 1 y from initiation of [177Lu]Lu-PSMA, but without association with the number of treatment cycles (12). Follow-up after the last cycle of treatment in our study was short, precluding definitive statements on possibly delayed renal toxicity.
In the present study, the overall incidence of grades 3–4 adverse events was 32.4%, and the adverse event rate leading to dose reduction was 15.3%. In the CARD study, the rate of grades 3–4 adverse events was 56.3% in patients receiving cabazitaxel as a second-line chemotherapy (13). Cabazitaxel showed a higher frequency of dose reductions (cabazitaxel arm of TheraP trial, 20.8%; CARD trial, 21.4%) (10,13). In our study, the rate of adverse events resulting in dose reduction was relatively higher in patients who had not received docetaxel or cabazitaxel previously. Patients with comorbidities unsuitable for chemotherapy may be more likely to experience a higher rate of adverse events, leading to a dose reduction. Furthermore, we observed decreased rates of dose reduction in patients who received docetaxel or cabazitaxel (11.1%) or both (12.1%) before extended [177Lu]Lu-PSMA therapy compared with the rates reported in the CARD study (21.4%) (13). This suggests that extended [177Lu]Lu-PSMA therapy has a favorable safety profile compared with second-line cabazitaxel therapy.
OS from the initiation of [177Lu]Lu-PSMA was 23.2 mo for the continuous treatment group and 40.2 mo for the rechallenge group. In both groups, the OS was considerably longer than that observed in the phase 3 VISION study (1). Notably, our patient cohort has a significant selection bias compared with the VISION study as only patients who showed response after 6 cycles were eligible for extended treatment.
In our rechallenge subgroup, [177Lu]Lu-PSMA therapy resulted in high biochemical response rate (first rechallenge, 37.1%; second rechallenge, 75.0%) as well as a favorable OS of 40.2 mo. These results are comparable with those of a previous study on 30 patients under [177Lu]Lu-PSMA rechallenge treatment. In that study, Yordanova et al. demonstrated a biochemical response in almost 40% of patients after 2 cycles of rechallenge, and an OS of 25 mo from the first cycle (3). Interestingly, the biochemical response rate after the first rechallenge treatment in our [177Lu]Lu-PSMA-retreated group (37.1%) was comparable to that of patients receiving cabazitaxel for the first time (36%) in the presence of progressive disease with failure of previous treatments including enzalutamide or abiraterone and docetaxel (13). Rechallenge therapy with docetaxel was previously studied in a phase 3 trial for mCRPC patients who progressed to mCRPC and previously received docetaxel in a metastatic hormone-sensitive prostate cancer setting (14). Only 14% of patients in the docetaxel rechallenge arm experienced a 50% PSA decline (14). Additionally, the docetaxel rechallenge did not prolong the OS of mCRPC patients who responded to the first-line docetaxel therapy when compared with OS in non–taxane-based therapy (15). The cabazitaxel rechallenge has also been shown to be feasible and to achieve a median OS of 51 mo from the start of the first dose of cabazitaxel, which is longer than the 40 mo reported here for the [177Lu]Lu-PSMA rechallenge group (16).
Given the limited treatment options in mCRPC, extension or rechallenge treatment with substantial antitumor effects will be increasingly discussed for [177Lu]Lu-PSMA. Therefore, the evaluation of the safety of [177Lu]Lu-PSMA beyond 6 cycles is important (17). Currently, several ongoing clinical trials are evaluating [177Lu]Lu-PSMA treatment in taxane-naïve patients, for example, PSMAfore (NCT04689828), SPLASH (NCT04647526), ECLIPSE (NCT05204927), PSMAddition (NCT04720157), and UpfrontPSMA (NCT04343885). These patients will likely experience disease progression later on and might be candidates for rechallenge with [177Lu]Lu-PSMA.
Our retrospective analysis comes with limitations. We report here the results of extended treatment with [177Lu]Lu-PSMA in selected patients with either macroscopic residual disease under stable or partial response in the continuous group or excellent initial disease reduction allowing a therapeutic pause in the retreated group. Selection criteria introduce bias toward favorable survival. The results may, therefore, not be representative of patients outside these clinical scenarios. In addition, the assessment of efficacy and safety is less accurate in a retrospective design than in a prospective one because of a lower level of control and follow-up. In particular, the follow-up period after the last treatment cycle was short, precluding definitive statements about potentially delayed renal toxicity.
CONCLUSION
Patients with mCRPC who have a favorable initial response to [177Lu]Lu-PSMA therapy may benefit from extended treatment beyond 6 cycles. Extended treatment was associated with favorable safety and substantial biochemical response. The benefit of this treatment option has yet to be evaluated in prospective, randomized, and controlled trials.
DISCLOSURE
Tugce Telli declares Abx (speaker) fees outside the submitted work. Robert Seifert has received support from the Else Kröner-Fresenius-Stiftung and the Boehringer Ingelheim Fonds. Constantin Lapa reports prior consulting activities for Blue Earth Diagnostics Ltd. and Novartis outside the submitted work. Wolfgang Weber reports fees from Blue Earth Diagnostics Ltd. (consultant, research support), ITM (research support, consultant), RayzeBio (research support, consultant), Eckert-Ziegler (research support, speaker), Roche (research support, consulting), and Siemens Healthineers (research support). Boris Hadaschik is on advisory boards for Janssen, Bayer, ABX, Lightpoint, Amgen, MSD, Pfizer, and Novartis; is an invited speaker for Accord, Astellas, and Janssen R&D; received honoraria from Uromed; received research funding from AAA/Novartis, Bristol Myers Squibb, and German Research Foundation; and has leadership roles for DKG AUO and DGU. Ken Herrmann reports consultant fees from Advanced Accelerator Applications, a Novartis company, Amgen, AstraZeneca, Bain Capital, Bayer, Boston Scientific, Convergent, Curium, Debiopharm, EcoR1, Fusion, GE Healthcare, Immedica, Isotopen Technologien München, Janssen, Merck, Molecular Partners, NVision, POINT Biopharma, Pfizer, Radiopharm Theranostics, Rhine Pharma, Siemens Healthineers, SOFIE Biosciences, Telix, Theragnostics, and ymabs; received research grants from Advanced Accelerator Applications, a Novartis company, Boston Scientific, and Janssen; and has stock or other ownership interests with AdvanCell, Aktis Oncology, Convergent, NVision, Pharma 15, and SOFIE Biosciences. Kambiz Rahbar reports consulting fees from ABX, ABX-CRO, Bayer Healthcare, Pharmtrace, and AAA/Novartis and lectureship payments from AAA, Bayer Healthcare, Janssen Cilag, Sirtex, and Amgen. Matthias Eiber reports fees from Blue Earth Diagnostics Ltd. (consultant, research funding), Novartis/AAA (consultant, speaker), Telix (consultant), Bayer (consultant, research funding), RayzeBio (consultant), Point Biopharma (consultant), Eckert-Ziegler (speaker), Janssen Pharmaceuticals (consultant, speaker’s bureau), Parexel (image reviews), and Bioclinica (image review) outside the submitted work and a patent application for rhPSMA. Wolfgang Fendler reports fees from SOFIE Bioscience (research funding), Janssen (consultant, speaker), Calyx (consultant, image review), Bayer (consultant, speaker, research funding), Novartis (speaker, consultant), Telix (speaker), GE Healthcare (speaker), Eczacıbaşı Monrol (speaker), Abx (speaker), and Amgen (speaker) outside the submitted work. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is [177Lu]Lu-PSMA therapy extension, either with continuous or rechallenge methods, safe? Does therapy extension improve survival and provide benefits?
PERTINENT FINDINGS: Extended therapy with [177Lu]Lu-PSMA has been shown to be safe and has not been associated with an increased incidence of grades 3–4 toxicity. Patients treated with extended treatment experience a favorable median OS of 31.3 mo from the first administration. The response to [177Lu]Lu-PSMA rechallenge demonstrates the preserved efficacy of [177Lu]Lu-PSMA after a treatment break.
IMPLICATIONS FOR PATIENT CARE: Patients receiving extended [177Lu]Lu-PSMA therapy experienced good responses and had long OS. Extended treatment was well tolerated.
Footnotes
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 22, 2023.
- Revision received March 25, 2024.