Visual Abstract
Abstract
177Lu-PSMA therapy is an effective treatment in patients with metastatic castration-resistant prostate cancer. SUVmean is a valuable screening biomarker to assess the suitability for 177Lu-PSMA therapy but requires quantitative software. This study aims to develop a simple, clinically applicable prostate-specific membrane antigen PET/CT score that encompasses the elements of SUVmean without requiring additional quantification. Methods: Datasets from ethics-approved trials of patients with metastatic castration-resistant prostate cancer after androgen receptor signaling inhibition and taxane chemotherapy (or unfit for taxane), who were treated with 177Lu-PSMA-617 and 177Lu-PSMA I&T with a pretreatment screening with 68Ga-PSMA-11 PET/CT, and clinical outcome data, including a prostate-specific antigen (PSA) 50% response rate (PSA50), PSA progression-free survival (PSA-PFS), and overall survival (OS), were included. The screening 68Ga-PSMA-11 PET/CT of all participants was analyzed both semiquantitatively and visually. Semiquantitative analysis was used to derive the SUVmean. Visual analysis of the 68Ga-PSMA-11 PET/CT images involved a binary visual heterogeneity assessment (homogeneous or heterogeneous), allocating a tumor SUVmax range (<15, 15–29, 30–49, 50–79, or ≥80). A 4-category score incorporating both heterogeneity and intensity of tumors (HIT) was then developed as a combination of heterogeneity and intensity (SUVmax range). The SUVmax was less than 15 for score 1, 15–79 with heterogeneous intensity for score 2, 15–79 with homogeneous intensity for score 3, and 80 or greater for score 4. This score was evaluated according to clinical outcomes (PSA50, PSA-PFS, and OS) and compared with SUVmean. Results: Data from 139 participants were analyzed. In total, 75 (54%) patients achieved a PSA50 with a median PSA-PFS of 5.5 mo (95% CI, 4.1–6.0 mo) and an OS of 13.5 mo (95% CI, 11.1–17.9 mo). SUVmean was associated with PSA50 and survival outcomes when analyzed as a continuous variable or as quartiles. The PSA50 for HIT scores 1–4 was 0%, 39%, 65%, and 76%, respectively. The HIT score was strongly related to PSA-PFS and OS (log-rank test, P < 0.001 and P = 0.002). The median PSA-PFS for HIT scores 1–4 was 1.0, 4.1, 6.0, and 8.5, respectively, and the median OS was 7.6, 12.0, 18.5, and 16.9 mo, respectively. Cohen κ between readers for the HIT score was 0.71. Conclusion: A prostate-specific membrane antigen PET/CT score incorporating HIT derived from tools on a standard PET workstation is comparable with quantitative SUVmean as a prognostic tool following 177Lu-PSMA therapy.
Treatment with 177Lu-PSMA-617 improves the overall survival (OS) in men with metastatic castration-resistant prostate cancer (mCRPC) after androgen signaling inhibition and taxane chemotherapy (1). 177Lu-PSMA is well tolerated and shows improved quality of life compared with second-line chemotherapy (2). Despite this, approximately one third of patients will have upfront treatment resistance or a limited duration of response to 177Lu-PSMA radiopharmaceutical therapy (3). Developing imaging biomarkers to better predict response is important to further improve patient outcomes. The TheraP and VISION trials found that semiquantitatively derived SUVmean from prostate-specific membrane antigen (PSMA) PET/CT imaging is predictive of treatment response with 177Lu-PSMA-617 (4–6). However, deriving SUVmean requires dedicated software programs not currently clinically available. The aim of this study is to develop a reproducible assessment method using standard PET workflow tools that correlates with SUVmean and is predictive of patient outcomes with 177Lu-PSMA.
MATERIALS AND METHODS
Study Population
This study is a retrospective analysis of screening 68Ga-PSMA-11 PET/CT parameters and patient outcomes including prostate-specific membrane (PSA) 50% response rate (PSA50), PSA progression-free survival (PSA-PFS), and OS from 3 previously published clinical trials in men with progressive mCRPC who were undergoing 177Lu-PSMA therapy after at least 1 line of androgen receptor signaling inhibition and 1 line of taxane chemotherapy or who were determined ineligible for taxane chemotherapy (7–9). The institutional review board of St. Vincent’s Hospital approved this retrospective study (Human Research Ethics Committee approval number 2022/ETH00924), and the requirement to obtain informed consent was waived.
PSMA PET/CT Acquisition and Visual Analysis
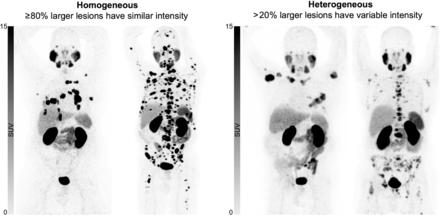
68Ga-PSMA-11 PET/CT imaging was undertaken per institutional or clinical trial protocols before treatment with 177Lu-PSMA. Images were analyzed both visually and semiquantitatively. Visual assessment included evaluation of heterogeneity and tumor intensity relative to parotid and liver avidity on rotating 3-dimensional maximum-intensity-projection images adjusted to the SUV window range (0–15). First, visual analysis was performed by 3 experienced nuclear medicine specialists who were masked to the clinical outcomes. Heterogeneity was a binary score. If at least 80% of all lesions not impacted by partial-volume effects (larger lesions) had similar intensities, this was classified as homogeneous. If more than 20% of larger lesions had variable intensity (inter- or intralesional), this was classified as heterogeneous (Fig. 1). Second, the readers measured the SUVmax of the most intense lesions and allocated an SUVmax range (<15, 15–29, 30–49, 50–79, or ≥80). Third, the readers evaluated if the most intense lesions were above the parotid intensity or between the liver and parotid intensities. No patients in whom the highest lesional intensity was below the liver intensity were enrolled. All readers participated in a 30-min training session that involved an explanation of the heterogeneity binary assessment and a consensus read of 20 68Ga-PSMA-11 PET/CT scans outside the study dataset. The heterogeneity category given by most readers was used for analysis.
Maximum-intensity projection of PSMA PET/CT showing 2 patients with homogeneous PSMA uptake in lesions (left) and 2 patients with heterogeneous PSMA uptake in lesions (right).
PSMA PET/CT Quantitative Analysis
Semiautomated segmentation of baseline 68Ga-PSMA-11 PET/CT was performed using MIM software (LesionID; MIM Software Inc.) and a standardized semiautomated workflow to delineate regions of interest with a minimum SUVmax cutoff of 3 and a lesion size of at least 0.5 mm. All lesions identified quantitatively were reviewed by experienced nuclear medicine physicians. Output parameters included SUVmean, SUVmax, and total tumor volume.
Interreader Reliability
After development of the heterogeneity-and-intensity-of-tumors (HIT) score, a full reread of the same dataset was performed by an additional 2 experienced nuclear medicine specialists using HIT scores 1–4 with comparison of Cohen κ between these readers.
Clinical Outcomes
All patients were treated with 177Lu-PSMA until they were no longer clinically benefiting. Clinical outcomes included PSA50, PSA-PFS, and OS. PSA50 was defined as a PSA decline of 50% or more compared with baseline at any time during the treatment. PSA-PFS was defined as the time from treatment initiation to PSA progression or death from any cause. PSA progression was defined as at least a 25% increase in PSA of at least 2.0 ng/mL above nadir per the criteria of the Prostate Cancer Clinical Trials Working Group 3 (10). OS was defined as the time from treatment initiation to death from any cause.
Statistical Analysis
The analysis followed 3 main phases. First, SUVmean was confirmed as a predictor of outcomes in the sample by entering it continuously as a restricted cubic spline function with knots at the tertiles into a logistic (for PSA50) or Cox regression (for PSA-PFS and OS) and plotting the results. In the Cox regression, the (near) median value of 8 was set as the reference, and analysis time began on the date of cycle 1. Kaplan–Meier plots were also generated with SUVmean entered as quartiles and log-rank tests used to identify differences in the survival curves. Second, the relation between SUVmean and a combination of SUVmax range and visual heterogeneity was examined. Nested linear regression models and the likelihood ratio test were used to assess whether heterogeneity, above the SUVmax range alone, added significantly to the model fit predicting SUVmean. A scatterplot with locally weighted regression curves of log-transformed SUVmean versus SUVmax range, by heterogeneity, was generated to visually guide the creation of the 4-category HIT score. HIT score 1 was derived separately from the scatterplot on the basis of data demonstrating no PSA response in patients with an SUVmax less than 15 (8). HIT scores 2–4 were derived on the basis of the scatterplot. Score 4 required an SUVmax of at least 80 and was derived from the scatterplot as a point above which heterogeneous or homogeneous curves joined with no significant differences between the 2 groups—and with higher treatment responses. Finally, the relation between the 4-category HIT score and outcomes was assessed similarly to quartiles of SUVmean. The predictive power of the Cox survival models including the HIT score, the quartiles of SUVmean, or the quartiles of SUVmax range was quantified with the Somers D statistic. Exploratory analysis assessed the survival outcomes according to tumor intensity relative to parotid intensity with Kaplan–Meier plots and log-rank tests, and interrater agreement of the HIT score was calculated on the basis of image readings by 2 readers once the HIT score had been created. Analysis was performed with Stata/MP version 17.0 (StataCorp LLC), and tests were 2-sided with significance set at less than 0.05.
RESULTS
Patient Characteristics
In total, 139 patients who had received 177Lu-PSMA-617 or 177Lu-PSMA I&T in two phase 2 clinical trials and a published clinical therapy program between 2016 and 2022 were included in this analysis (Table 1). All patients received a median of 4 doses of 7.5 GBq of 177Lu-PSMA. The overall PSA50 was 54%, the number of PSA-PFS events was 120 with a median PSA-PFS of 5.5 mo (95% CI, 4.1–6.0 mo), and the number of deaths was 82, with a median OS of 13.5 mo (95% CI, 11.1–17.9 mo).
Patient Characteristics
SUVmean
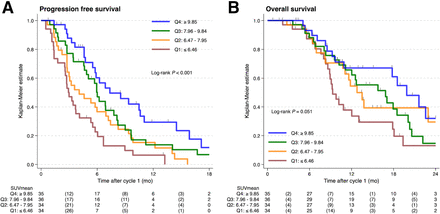
The median semiquantitatively derived SUVmean was 8.0 (interquartile range, 6.6–9.9). Increasing SUVmean as a continuous variable demonstrated a strong relationship with a higher probability of PSA50 and lower hazard ratio of PSA-PFS and OS, evidenced by a near-monotonic function with these outcomes (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). When assessed as quartiles, SUVmean significantly predicted PSA-PFS (P < 0.001) with borderline significance for OS (P = 0.051) (Fig. 2).
Kaplan–Meier curve (log-rank tests) of PSA-PFS (A) and OS (B) for semiquantitative SUVmean quartiles (Q1–Q4).
HIT Score
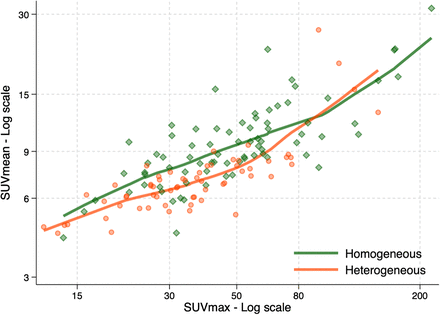
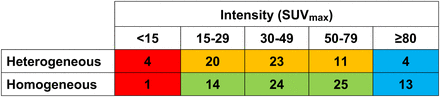
The 4-category HIT score was derived from an evaluation of heterogeneity and allocation of an SUVmax range. Inclusion of heterogeneity significantly improved the prediction of SUVmean above that of the SUVmax range (likelihood ratio test, P = 0.0041); hence, both factors were required. On evaluation of the scatterplot and locally weighted regression curves (Fig. 3), in association with results from previous studies, the following categories were devised: score 1 was all patients with an SUVmax of less than 15; score 2 included patients defined as having heterogeneous disease visually with an SUVmax between 15 and 79; score 3 included patients with visually homogeneous disease and an SUVmax between 15 and 79; score 4 included all patients with an SUVmax of at least 80 independent of whether the tumor was homogeneous or heterogeneous (Fig. 4). The distribution of the HIT score among the sample was as follows: score 1, n = 5 (3.6%); score 2, n = 54 (39%); score 3, n = 63 (45%); and score 4, n = 17 (12%).
Scatterplot of weighted regression curves of log SUVmean vs. SUVmax range, by visual heterogeneity score (homogeneous vs. heterogeneous).
Color-coded table of HIT scores 1–4 incorporating SUVmax range (most intense lesion) and binary visual heterogeneity with patient numbers in each group. Red = HIT score 1; yellow = HIT score 2; green = HIT score 3; blue = HIT score 4.
HIT-Score Agreement
Following HIT-score development, images were read by 2 readers and a score was assigned. The interrater agreement (Cohen κ) of the HIT score was 0.71 (95% CI, 0.60–0.82), and the percentage agreement was 82%.
HIT Score and Outcomes
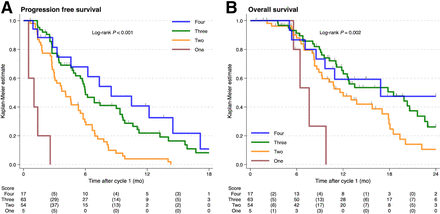
The PSA50 for a HIT score of 1–4 was 0% (0/5), 39% (21/54), 65% (41/63), and 76% (13/17), respectively. The HIT score statistically significantly predicted both PFS and OS (log-rank test, P < 0.001 and P = 0.002, respectively) (Fig. 5). The differences in survival curves between scores 2 and 3 (same SUVmax range but heterogeneous vs. homogeneous) were also significant for PFS (P < 0.001) and OS (P = 0.040). The median PFS (95% CI) for HIT scores 1–4 was 1.0 mo (0.6 mo to not estimable), 4.1 mo (2.9–5.5 mo), 6.0 mo (5.1–9.4 mo), and 8.5 mo (3.3–14.5 mo), respectively. The corresponding median OS (95% CI) was 7.6 mo (5.5 mo to not estimable), 12.0 mo (8.9–17.9 mo), 18.5 mo (12.0–21.6 mo), and 16.9 mo (7.1 mo to not reached). Cox models with a HIT score had predictive power comparable to that of SUVmean quartiles for PSA-PFS (Somers D of 0.25 vs. 0.27) and OS (Somers D of 0.15 vs. 0.16) and exceeded those for SUVmax range quartiles (0.17 for PFS and 0.12 for OS).
Kaplan–Meier curve (log-rank tests) of PSA-PFS (A) and OS (B) for HIT scores 1–4.
Physiologic Activity and Heterogeneity
Most patients had lesion intensity that was greater than parotid intensity, n = 126 (91%), with the remainder having an intensity between those of the liver and parotid. No statistically significant difference in survival curves for PSA-PFS or OS between those with intensity above or below that of the parotid was observed (Supplemental Fig. 2).
DISCUSSION
Developing screening imaging biomarkers that can predict response to 177Lu-PSMA is important to improve patient outcomes and to better personalize treatment options. Quantitative whole-body SUVmean has shown value in predicting treatment response in men treated with 177Lu-PSMA in both TheraP and VISION trials; however, the clinical application of this tool is limited by the onerous requirements for image quantitation (4,5). This study has found that the HIT score, derived using tools available on clinical PET workstations, shows promising predictive capability for both PFS and OS in men being treated with 177Lu-PSMA therapy.
SUVmean is a semiquantitatively derived calculation of the mean voxel intensity of total-body tumor deposits. It gives a good measure of both the intensity of PSMA expression in tumor deposits and the variability of PSMA expression intra- and intertumorally. A patient with heterogeneous PSMA expression may have a low SUVmean despite some deposits demonstrating high PSMA expression. Metastatic prostate cancer is inherently heterogeneous, with PSMA expression shown to be variable both within and between tumoral deposits (11). This heterogeneity could significantly impact the response to 177Lu-PSMA (12). The HIT score using the most intense lesion SUVmax ranges with a binary visual tumor heterogeneity scoring on 68Ga-PSMA PET/CT screening successfully predicts both PSA-PFS and OS in response to 177Lu-PSMA therapy in men with mCRPC, with comparable predictability to quantitative SUVmean quartiles.
Several previous studies have shown that PSMA SUVmax is not predictive of treatment response to 177Lu-PSMA (4,13–15). However, these studies used PSMA SUVmax as a continuous or binary variable (SUVmax < 20 and ≥ 20). In this study, 5 ranges of PSMA SUVmax (<15, 15–29, 30–49, 50–79, and ≥80) were used. This was to allow better separation of intensity levels between patients and to reduce the impact of inherent limitations of SUVmax reproducibility. SUVmax is dependent on several variables that limit its reproducibility such as variation in body habitus, size of the maximal voxel between different scanners, and the statistical quality of the images (16). The HIT score, through combining a binary visual heterogeneity evaluation and SUVmax range, optimizes the clinical value of SUVmax, while minimizing the limitations of reproducibility.
The PSMA PET/CT tumor–to–salivary gland ratio proposed a visual method for assessment of tumor intensity using parotid intensity. A cohort of 237 patients was classified as high (>80% lesions above parotid), intermediate, and low (>80% lesions below parotid) (13). That study found that a high tumor-to–salivary gland ratio had higher PSA50 (63% vs. 17%) and longer PSA-PFS and OS than did the low tumor-to–salivary gland ratio (6.7 vs. 1.9 mo and 14.3 vs. 12.9 mo, respectively) (13). The current study shows no significant difference in patient outcome using PSMA intensity of the most active lesion above or below the parotid intensity but did not compare parotid with tumor intensity at all sites. Using whole-body lesions in tumor–to–salivary gland ratios provided an indirect measure of tumor heterogeneity and thus allowed stratification. HIT score integrating SUVmax ranges and binary heterogeneity score significantly correlates with PSA50 and both PSA-PFS and OS with higher separation between the highest and lowest scores in PSA50, PSA-PFS, and OS (76% vs. 0%, 8.5 vs. 1.0 mo, and 16.9 vs. 7.6 mo, respectively).
The current study has several limitations. Analysis of the screening 68Ga-PSMA-11 PET/CT was undertaken retrospectively using data from single-institution trials. Results will need to be reproduced and validated in more diverse clinical datasets before clinical implementation, including full assessment of the reproducibility of the HIT score between multiple readers. Screening criteria for trial enrollment required minimal levels of PSMA intensity on 68Ga-PSMA-11 PET/CT images; hence, the number of patients with an SUVmax less than 15 (HIT score 1) is low. 68Ga-PSMA-11 was the only PET radiopharmaceutical used for screening patients in this study. The use of a heterogeneity score should be applicable across PSMA PET/CT ligands, and the use of the SUVmax range rather than using absolute SUVmax may mitigate differences between PSMA ligands, but this needs separate evaluation for confirmation.
CONCLUSION
A PSMA PET/CT score incorporating the HIT score derived from tools on a standard PET workstation is comparable to SUVmean as a prognostic tool for PFS and OS following 177Lu-PSMA therapy without the need for total-body quantitation. Further studies are warranted to validate the clinical utility of the HIT score.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can a visually based score using standard PET workstation tools predict patient outcomes with 177Lu-PSMA therapy on PSMA PET/CT screening and correlate with semiquantitative SUVmean?
PERTINENT FINDINGS: The HIT score using standard PET workstation tools to measure tumor PSMA intensity and heterogeneity predicted PSA50, PSA-PFS, and OS and was comparable to quantitative SUVmean.
IMPLICATIONS FOR PATIENT CARE: The HIT score is a simple clinically applicable PSMA PET/CT score that shows promising capability to predict patient outcomes with 177Lu-PSMA therapy without requiring further quantification.
ACKNOWLEDGMENTS
We thank the patients and the clinical staff at the Department of Theranostics and Nuclear Medicine at St. Vincent’s Hospital, Sydney, for their support.
Footnotes
Published online Apr. 18, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication November 5, 2023.
- Revision received March 15, 2024.