Visual Abstract
Abstract
Response Evaluation Criteria in Prostate-Specific Membrane Antigen Imaging (RECIP) 1.0 is an evidence-based framework to evaluate therapeutic efficacy in metastatic prostate cancer using prostate-specific membrane antigen (PSMA) PET/CT. This study aimed to evaluate the associations of interim PSMA PET/CT by RECIP 1.0 with short-term outcome after radiopharmaceutical treatment. Methods: This multicenter retrospective study included patients with metastatic castration-resistant prostate cancer who underwent [177Lu]Lu-PSMA radiopharmaceutical therapy at 3 academic centers and received PSMA PET/CT at baseline and at 12 wk. Pairs of PSMA PET/CT images were assessed by 5 readers for visual RECIP 1.0. The primary outcome was the association of RECIP with prostate-specific antigen progression-free survival (PSA-PFS) by Kaplan–Meier analysis. Results: In total, 124 of 287 screened patients met the inclusion criteria, with 0 (0%), 29 (23%), 54 (44%), and 41 (33%) of those 124 patients having complete response, partial response, stable disease, or progressive disease (PD) by visual RECIP 1.0, respectively. Patients with visual RECIP PD had a significantly shorter PSA-PFS than those with RECIP stable disease or with RECIP partial response (2.6 vs. 6.4 vs. 8.4 mo; P < 0.001). The median PSA-PFS among patients with RECIP PD versus those with non-RECIP PD was 2.6 versus 7.2 mo (hazard ratio, 13.0; 95% CI, 7.0–24.1; P < 0.001). Conclusion: PSMA PET/CT by RECIP 1.0 after 2 cycles of [177Lu]Lu-PSMA is prognostic for PSA-PFS. PSMA PET/CT by RECIP 1.0 may be used in earlier stages of prostate cancer to evaluate drug efficacy and to predict progression-free survival.
- metastatic castration-resistant prostate cancer
- PSMA PET
- response evaluation
- RECIP
- LuPSMA
- radiopharmaceutical therapy
Prostate-specific membrane antigen (PSMA) theranostics with [177Lu]Lu-PSMA-617 improves the overall survival (OS) and progression-free survival in patients with metastatic castration-resistant prostate cancer (mCRPC) (1), which has led to drug approval. The U.S. Food and Drug Administration approved [68Ga]Ga-PSMA-11, [18F]DCFPyL, and [18F]rhPSMA-7.3 PET/CT in patients with prostate cancer and suspected metastases who were candidates for initial definitive therapy or who had suspected recurrence based on elevated prostate-specific antigen (PSA) levels to determine eligibility for [177Lu]Lu-PSMA-617 therapy (2–4).
Besides staging and restaging, cancer imaging can be used to evaluate therapeutic efficacy. Objective criteria for measuring response to cancer treatment are critical to clinical research and practice (5). Therapeutic clinical trials of metastatic disease often use radiographic endpoints to evaluate response to treatment (6).
Response Evaluation Criteria in PSMA Imaging (RECIP) version 1.0 is an evidence-based framework to evaluate therapeutic efficacy in metastatic prostate cancer using PSMA PET/CT and was developed on the basis of OS outcomes in patients treated with [177Lu]Lu-PSMA (7–9). Further studies validated RECIP 1.0 for measuring response based on associations with OS in mCRPC patients who received androgen-receptor–signaling inhibitors (10,11) and in early-stage prostate cancer patients with biochemical recurrence after the initial therapy (12).
PSA progression-free survival (PSA-PFS) is an efficacy endpoint commonly used in metastatic prostate cancer as a surrogate for OS. Currently, evidence is lacking for the association of RECIP 1.0 with short-term outcome, that is, progression-free survival.
The current retrospective analysis aims to evaluate the associations of 12-wk PSMA PET/CT by RECIP 1.0 with progression-free survival in mCRPC patients who receive [177Lu]Lu-PSMA.
MATERIALS AND METHODS
Patients
Consecutive patients with mCRPC who were treated with [177Lu]Lu-PSMA-617 or [177Lu]Lu-PSMA imaging and therapy (I&T) between December 10, 2014, and July 19, 2019, at 3 academic medical centers were screened for eligibility. Eligible patients had received PSMA PET/CT at baseline and at 12 wk after 2 cycles of treatment (interim PET) using the same PSMA-targeting radiotracer for the baseline and interim PET examinations. Detailed inclusion and exclusion criteria are given in Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org). Treatment protocols are detailed in the supplemental materials. All patient data were previously reported (7–9). These prior publications reported the development of RECIP 1.0 and evaluated its prognostic value for OS. In contrast to prior work, this study investigated the prognostic value of RECIP 1.0 for PSA-PFS after [177Lu]Lu-PSMA radiopharmaceutical therapy. This retrospective analysis was approved by the ethics committee of each participating site (115/18S, 20-000954, and UKE 19-8570-BO), and the requirement for study-specific consent was waived. PSMA PET/CT image acquisition protocols were described previously (7) and are detailed in Supplemental Table 1.
Image Analysis
PSMA PET/CT images were interpreted independently by 5 experienced nuclear medicine physicians. Each reader was provided with guidelines for image interpretation (supplemental materials), was masked to the outcome data, and was not involved in the study design. Readers were asked to interpret the baseline and 12-wk posttreatment PSMA PET/CT scans for visual RECIP and quantitative RECIP 1.0, as described previously (9).
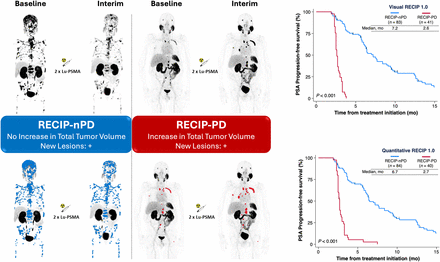
Visual RECIP was determined by combining changes in PSMA-positive total tumor volume evaluated visually by nuclear medicine physicians with the status of new lesions. Visual assessment of changes in PSMA-positive total tumor volume were approximated qualitatively by means of side-by-side comparison of baseline and follow-up maximum-intensity projection PSMA PET/CT images. In borderline cases, additional analysis of axial sections was performed.
Quantitative RECIP was determined by combining changes in PSMA-positive total tumor volume determined by a nuclear medicine physician using tumor segmentation software (qPSMA software (13)) with the status of new lesions. After tumor segmentation was performed, the PSMA volume was obtained by calculating the volume of all voxels that were annotated as PSMA-positive tumors. Changes in total tumor volume were determined by calculating the percentage change between baseline and follow-up PSMA PET/CT scans.
Disagreement among readers was resolved by majority rule. Definition of RECIP 1.0, including definition of occurrence of new lesions, is given in Table 1.
Definitions of New Lesions and RECIP
Statistical Analysis
Values are reported as medians with interquartile ranges for continuous variables and numbers with percentages for categoric variables. Response according to RECIP 1.0 was classified into progressive disease (PD), stable disease (SD), partial response (PR), or complete response and dichotomized for the differentiation of progression versus nonprogression (RECIP PD vs. non-PD, where non-PD included complete response, PR, and SD). The primary outcomes of our study were the associations of RECIP 1.0 with PSA-PFS. Associations between RECIP 1.0 and PSA-PFS were tested by Cox regression analyses, and the hazard ratio (HR), its 95% CI, and the corresponding P values were derived. PSA progression was defined per Prostate Cancer Clinical Trial Working Group 3 criteria as the time from treatment initiation to PSA progression (≥25% increase from baseline). The median survival time and its 95% CI for each group of patients and the entire cohort were calculated using Kaplan–Meier analysis. Kaplan–Meier curves were truncated when the number at risk fell below 10. Subgroup analyses were performed to evaluate associations of RECIP 1.0 with PSA-PFS for each treatment type, that is, [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA I&T. A P value of less than 0.05 was considered indicative of a statistically significant difference. All statistical analyses were performed using SPSS Statistics, version 27 (IBM).
RESULTS
In total, 124 of 287 (43%) screened patients with mCRPC were eligible and included. Of the 124 eligible patients, 102 (82%) received treatment with [177Lu]Lu-PSMA I&T and 22 (18%) received treatment with [177Lu]Lu-PSMA-617. The median age was 73 y (interquartile range, 67–76 y). In total, 99 of 124 (80%) patients received taxane-based chemotherapy and 123 of 124 (99%) received androgen-receptor–signaling inhibitors. Detailed patient characteristics are given in Table 2. The data cutoff date for the final analysis was July 1, 2022, and all patients had PSA progression at the last follow-up. The median PSA-PFS was 3.8 mo (95% CI, 3.1–4.6 mo). After the majority rule of the 5 readers for visual RECIP 1.0 was applied, 41 of 124 (33%) patients had RECIP PD and 83 of 124 (67%) had RECIP non-PD, of whom 0 (0%), 29 (23%), and 54 (44%) of the 124 patients had visual RECIP complete response, PR, and SD, respectively. After the majority rule for quantitative RECIP 1.0 was applied, 40 of 124 (32%) patients had RECIP PD and 84 of 124 (68%) had RECIP non-PD, of whom 0 (0%), 40 (32%), and 44 (36%) of the 124 patients had visual RECIP complete response, PR, and SD, respectively.
Patient Characteristics (n = 124)
PSA-PFS
Visual RECIP
The median PSA-PFS among patients with RECIP PD versus RECIP SD versus RECIP PR was 2.6 versus 6.4 versus 8.4 mo, respectively (Fig. 1). RECIP PD was associated with a significantly shorter PSA-PFS than that with RECIP SD (HR, 11.2; 95% CI, 6.0–21.3; P < 0.001) and that with RECIP PR (HR, 17.1; 95% CI, 8.4–34.9; P < 0.001). RECIP SD was associated with shorter—albeit not statistically significant—PSA-PFS than that with RECIP PR (HR, 1.5; 95% CI, 0.9–2.5; P = 0.10). The median PSA-PFS among patients with RECIP PD versus RECIP non-PD was 2.6 versus 7.2 mo (HR, 13.0; 95% CI, 7.0–24.1; P < 0.001) (Fig. 1). In the subgroup analysis, RECIP PD was associated with shorter PSA-PFS than was RECIP non-PD in patients treated with [177Lu]Lu-PSMA I&T (HR, 12.7; 95% CI 6.4–25.1; P < 0.001) or [177Lu]Lu-PSMA-617 (HR, 10.5; 95% CI, 2.5–43.0; P = 0.001) (Supplemental Fig. 2).
Associations between visual RECIP 1.0 (A and B) and quantitative RECIP 1.0 (C and D) responses with PSA-PFS. nPD = non-PD.
Quantitative RECIP
The median PSA-PFS for RECIP PD versus that for RECIP SD versus that for RECIP PR was 2.7 versus 5.4 versus 8.9 mo, respectively (Fig. 1). RECIP PD was associated with significantly shorter PSA-PFS than were RECIP SD (HR, 4.7; 95% CI, 2.8–7.9; P < 0.001) and RECIP PR (HR, 10.7; 95% CI, 6.0–19.2; P < 0.001). RECIP SD was associated with significantly shorter PSA-PFS than was RECIP PR (HR, 2.9; 95% CI, 1.4–3.7; P < 0.001). The median PSA-PFS among patients with RECIP PD versus RECIP non-PD was 2.7 versus 6.5 mo (HR, 6.8; 95% CI, 4.1–11.2; P < 0.001) (Fig. 1). In the subgroup analysis, RECIP PD was associated with shorter PSA-PFS than was RECIP non-PD in patients treated with [177Lu]Lu-PSMA I&T (HR, 6.3; 95% CI, 3.7–10.9; P < 0.001) or [177Lu]Lu-PSMA-617 (HR, 10.5; 95% CI, 2.5–43.0; P = 0.001) (Supplemental Fig. 2).
DISCUSSION
OS is a gold standard endpoint in cancer research and is desired by regulatory authorities for drug approval in phase 3 registration trials. Surrogate endpoints are increasingly accepted by regulatory bodies for accelerated approvals of prostate cancer therapies, particularly in earlier disease stages, for example, metastatic hormone-sensitive prostate cancer, nonmetastatic CRPC, or early-stage mCRPC, in which the average life expectancy exceeds 2 y. Up to 48% of prostate cancer therapeutic trials use progression-free survival as a surrogate endpoint for OS (14). PSA-PFS is often used as a primary endpoint in phase 2 clinical trials of prostate cancer to investigate principal drug efficacy. For example, the EnzaP randomized trial of enzalutamide versus enzalutamide plus [177Lu]Lu-PSMA-617 in chemotherapy-naïve mCRPC patients used PSA-PFS as the primary endpoint (15), whereas the TheraP randomized trial of [177Lu]Lu-PSMA-617 versus cabazitaxel in postchemotherapy mCRPC patients used PSA-PFS as the secondary endpoint (16).
The present analysis found that response evaluation by RECIP 1.0 at 12-wk PSMA PET/CT is associated with progression-free survival after [177Lu]Lu-PSMA radiopharmaceutical therapy. Patients with RECIP PD had significantly shorter PSA-PFS than those patients with RECIP SD and RECIP PD. These findings suggest that PSMA PET/CT performed after 2 cycles of [177Lu]Lu-PSMA may identify patients who will shortly progress by PSA. A strategy in which patients with RECIP PD at 12 wk switch to a more efficacious treatment may improve clinical outcomes.
RECIP 1.0 is a framework for response evaluation that can be determined in two ways, that is, qualitatively by visual reads of nuclear medicine physicians and radiologists (visual RECIP) or quantitatively using tumor segmentation software (quantitative RECIP). A recent study found a 95% agreement between quantitative and visual RECIP PD versus non-PD. In the present analysis, visual RECIP PR failed to show significantly superior PSA-PFS compared with that of RECIP SD (HR, 1.5; P = 0.10), whereas the quantitative RECIP PR showed superior PSA-PFS compared with that of RECIP SD (HR = 2.9; P < 0.001), highlighting the need for training in evaluating responses in total tumor volume in PSMA PET/CT before applying visual RECIP 1.0 in clinical practice.
Altogether, previous and current findings demonstrated that RECIP 1.0 is associated with PSA-PFS and OS in mCRPC. The data support the implementation of RECIP 1.0 in daily practice and clinical trials for treatment response evaluation in mCRPC patients during [177Lu]Lu-PSMA radiopharmaceutical therapy. Notably, visual RECIP 1.0 should be used to determine only clinically relevant PD versus non-PD, whereas quantitative RECIP 1.0 can be used to classify PR versus SD versus PD. Further, the data encourage evaluation of associations of RECIP 1.0 with outcome data in patients who are earlier in the disease trajectory and who have a longer life expectancy.
The main limitation of this study is the use of the same patient cohort that was used to develop RECIP 1.0 (7). However, the current study used PSA-PFS as the study outcome, whereas the initial analysis used OS as the endpoint. Other limitations include the study’s retrospective design and lack of a comparative treatment arm to test the prognostic versus predictive value of RECIP 1.0.
CONCLUSION
PSMA PET/CT by RECIP 1.0 after 2 cycles of [177Lu]Lu-PSMA is prognostic for PSA-PFS. PSMA PET/CT by RECIP 1.0 may be used in earlier stages of prostate cancer to evaluate drug efficacy and to predict progression-free survival. Further studies to validate our findings in a prospective setting are warranted.
KEY POINTS
QUESTION: Is RECIP 1.0 associated with progression-free survival in patients with mCRPC who are treated with [177Lu]Lu-PSMA?
PERTINENT FINDINGS: This retrospective multicenter analysis demonstrated that interim PSMA PET/CT performed after 2 cycles of [177Lu]Lu-PSMA and evaluated by RECIP 1.0 is significantly associated with PSA-PFS.
IMPLICATIONS FOR PATIENT CARE: PSMA PET/CT by RECIP 1.0 may be used in earlier stages of prostate cancer to evaluate drug efficacy and predict progression-free survival.
DISCLOSURE
Andrei Gafita is supported by the Prostate Cancer Foundation (21YOUN18). Steven Rowe is a consultant for Progenics Pharmaceutical, Inc., a wholly owned subsidiary of Lantheus, Inc., and the licensee of 18F-DCFPyL. Loic Djaileb is the recipient of a grant from the Fondation ARC pour la recherche sur le cancer, Nuovo Soldati Foundation, and the Phillipe Foundation Inc. (New York). Boris Hadaschik is on the advisory boards of Janssen, Bayer, ABX, Lightpoint, Amgen, MSD, Pfizer, and Novartis; is an invited speaker for Accord, Astellas, and Janssen R&D; receives research funding from AAA/Novartis, Bristol Myers Squibb, and German Research Foundation; and has leadership roles for DKG AUO and DGU. Wolfgang Fendler reports fees from SOFIE Bioscience (research funding), Janssen (consultant, speaker), Calyx (consultant, image review), Bayer (consultant, speaker, research funding), Novartis (speaker, consultant), Telix (speaker), GE Healthcare (speaker), Eczacıbaşı Monrol (speaker), Abx (speaker), and Amgen (speaker) outside the submitted work. Ken Herrmann reports receiving consultant fees from Advanced Accelerator Applications, a Novartis company, Amgen, AstraZeneca, Bain Capital, Bayer, Boston Scientific, Convergent, Curium, Debiopharm, EcoR1, Fusion, GE Healthcare, Immedica, Isotopen Technologien München, Janssen, Merck, Molecular Partners, NVision, POINT Biopharma, Pfizer, Radiopharm Theranostics, Rhine Pharma, Siemens Healthineers, SOFIE Biosciences, Telix, Theragnostics, and ymabs; receiving research grants from Advanced Accelerator Applications, a Novartis company, Boston Scientific, and Janssen; and having stock or other ownership interests with AdvanCell, Aktis Oncology, Convergent, NVision, Pharma 15, and SOFIE Biosciences. Matthias Eiber reports fees from Blue Earth Diagnostics Ltd. (consultant, research funding), Novartis/AAA (consultant, speaker), Telix (consultant), Bayer (consultant, research funding), RayzeBio (consultant), Point Biopharma (consultant), Eckert-Ziegler (speaker), Janssen Pharmaceuticals (consultant, speakers bureau), Parexel (image review), and Bioclinica (image review) outside the submitted work and has a patent application for rhPSMA. He and other inventors are entitled to royalties on sales of POSLUMA. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Apr. 18, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 12, 2023.
- Revision received March 13, 2024.