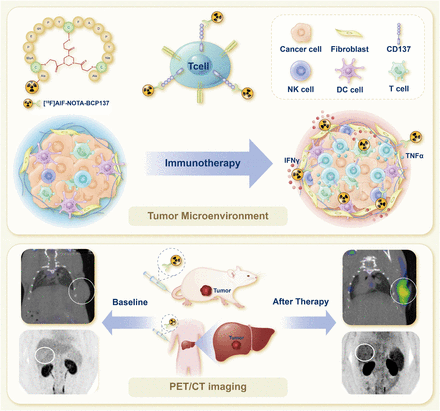
Visual Abstract
Abstract
Given the variability in the effectiveness of immune checkpoint blocking therapy among patients and tumor types, development of noninvasive methods for longitudinal assessment of immune cell function and early tumor response is crucial for precision immunotherapy. CD137 (4-1BB), a marker of activated T cells, plays a significant role in immunotherapy. However, its potential as an imaging biomarker for activated T cells in the tumor microenvironment has not been explored. This study introduces a bicyclic peptide–based probe that targets CD137 for noninvasive PET imaging of tumor-infiltrating activated T cells. Methods: A bicyclic peptide–based probe, [18F]AlF-NOTA-BCP137, was first designed and synthesized for quantitative and longitudinal whole-body visualization of CD137 dynamics. Initially, [18F]AlF-NOTA-BCP137 was assessed in mouse models with varying CD137 expression levels. Next, [18F]AlF-NOTA-BCP137 was used for longitudinal monitoring of systemic CD137 changes in a humanized tumor-bearing mouse model. Lastly, the probe was further evaluated in a small group of patients with hepatocellular carcinoma undergoing immunotherapy or combination immunotherapy. Results: [18F]AlF-NOTA-BCP137 PET accurately characterized CD137 expression in homologous transplanted mouse models and tumor patients. The findings from animal studies indicated that uptake of [18F]AlF-NOTA-BCP137 was predictive of the early therapeutic response to combination immunotherapies and was positively associated with the increased survival rates of mice with tumors. A preliminary clinical study involving small patient cohorts demonstrated that [18F]AlF-NOTA-BCP137 imaging effectively predicted early patient responses to immunotherapeutic interventions. Conclusion: [18F]AlF-NOTA-BCP137 PET imaging of CD137 is a promising and reliable method for evaluating the efficacy of multiple combination immunotherapies and merits further validation in larger-scale clinical trials. This approach has the potential for early noninvasive visualization of individual patient responses in combination cancer immunotherapy and will aid in tailoring personalized strategies for patients.
Footnotes
Published online Dec. 12, 2024.
- © 2025 by the Society of Nuclear Medicine and Molecular Imaging.
This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
SNMMI members
Login to the site using your SNMMI member credentials
Individuals
Login as an individual user