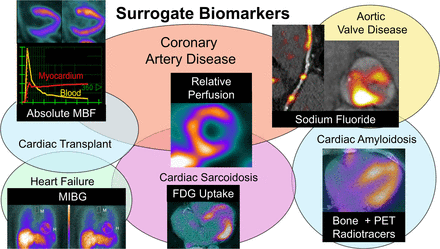
Visual Abstract
Abstract
Nuclear cardiology offers a diverse range of imaging tools that provide valuable insights into myocardial perfusion, inflammation, metabolism, neuroregulation, thrombosis, and microcalcification. These techniques are crucial not only for diagnosing and managing cardiovascular conditions but also for gaining pathophysiologic insights. Surrogate biomarkers in nuclear cardiology, represented by detectable imaging changes, correlate with disease processes or therapeutic responses and can serve as endpoints in clinical trials when they demonstrate a clear link with these processes. By providing early indicators of therapeutic efficacy—often before clinical outcomes manifest—surrogate biomarkers can accelerate treatment development. This disease-focused review will highlight key nuclear cardiology surrogate biomarkers, emphasizing the importance of standardized imaging protocols and robust quantitative techniques to ensure accuracy and reproducibility. We will also explore the challenges to the broader adoption of imaging biomarkers, including the need for well-defined pathophysiologic correlations, greater data diversity in clinical research, and overcoming regulatory barriers. Addressing these challenges will improve the utility of imaging biomarkers in clinical trials, enabling more precise cardiovascular care through early diagnosis and therapeutic monitoring, ultimately accelerating the development of novel cardiovascular therapies.
Nuclear cardiology encompasses a wide range of imaging tests that provide critical insights into myocardial perfusion, inflammation, metabolism, microcalcification, and other biologic processes (1). Given the extensive clinically relevant information it offers, nuclear cardiology plays a pivotal role in diagnosing and managing various cardiovascular diseases. Beyond its diagnostic utility, these imaging modalities can provide valuable pathophysiologic insights and serve as surrogate biomarkers in clinical trials. This review highlights key nuclear cardiology biomarkers, emphasizing their current applications and potential future roles in clinical trials.
DEFINING AND UTILIZING SURROGATE BIOMARKERS IN CLINICAL TRIALS
Biomarkers are measurable indicators that reflect biologic processes, disease states, or responses to therapeutic interventions. They can serve as tools for diagnosing, monitoring, or predicting the progression of diseases and the effectiveness of treatments. In nuclear cardiology, these biomarkers may include indicators such as changes in blood flow, myocardial metabolism, or other molecular targets. Surrogate imaging biomarkers are increasingly used in clinical trials as early indicators of therapeutic efficacy or disease progression, potentially replacing traditional clinical endpoints. Surrogate biomarkers can also be implemented in molecular targeting trials (trials designed to evaluate imaging agents or therapies that specifically target molecular processes), or in diagnostic trials (trials evaluating the ability of a diagnostic tool or imaging agent to quantify specific disease processes). Desired characteristics for specific trial designs are outlined in Table 1.
Summary of Desired Characteristics for Surrogate Biomarkers for Selected Trial Types*
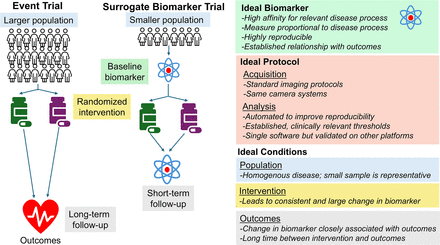
The design of a surrogate biomarker endpoint trial is similar to a traditional randomized controlled trial (Fig. 1), with patients randomized to different interventions and followed for changes in the biomarker rather than clinical events. In this type of trial, surrogate biomarker precision and test–retest reproducibility are critical. Using a surrogate biomarker endpoint allows researchers to detect changes in disease activity before clinical outcomes manifest. This can lead to smaller, shorter trials, reducing delays between disease processes and clinical events, and resulting in cost savings and quicker progress to phase III trials and regulatory approvals. It is also possible to use biomarkers as a method to enrich study populations with high-risk patients or those most likely to benefit from intervention. This approach can also lead to more efficient trial design but requires high target specificity to ensure patients are appropriately selected.
Comparison of event-based and surrogate biomarker endpoint trials. Ideal conditions for using surrogate imaging biomarker trial are outlined on left and include patient population (blue), baseline biomarker (green), intervention (yellow), follow-up biomarker (purple), and outcome (gray) considerations.
For a surrogate biomarker to be valid, it must meet specific criteria. The biomarker’s relationship to the disease process must be biologically plausible and closely linked to disease activity and severity, as well as meaningful clinical outcomes. Additionally, it should be reliable, reproducible, and quantifiable and should demonstrate sensitivity and specificity in measuring changes in disease activity/severity or therapeutic response. Biomarkers that do not meet these criteria may not improve trial efficiency or could lead to misleading results by overestimating or underestimating treatment effects.
KEY NUCLEAR CARDIOLOGY BIOMARKERS IN CLINICAL TRIALS
In this section, we explore the key nuclear cardiology imaging biomarkers that are playing, or are poised to play, a transformative role in clinical trial design. These biomarkers are essential for evaluating therapeutic strategies and understanding disease mechanisms in several cardiovascular disorders. Table 2 provides a summary of the selected nuclear cardiology biomarkers, the diseases they are applied to, and the potential therapies under investigation in clinical trials. Supplemental Table 1 showcases specific examples of trials where nuclear cardiology surrogate biomarkers have been successfully used (supplemental materials are available at http://jnm.snmjournals.org).
Major Surrogate Biomarkers in Nuclear Cardiology and Their Clinical Applications
Coronary Atherosclerosis
Relative Myocardial Perfusion
Stress myocardial perfusion imaging with SPECT or PET has long been a cornerstone of coronary artery disease diagnosis and management. Both modalities can quantify relative myocardial perfusion abnormalities for evaluating antiischemic therapies such as nitroglycerin and ranolazine (2), revascularization procedures, and treatments with potential myocardial injury risk (e.g., alcohol septal ablation) (3). These measures have a clear pathophysiologic relationship to the disease, and improvements in relative perfusion markers have been associated with decreased cardiovascular risk (4).
Relative perfusion has been used to assess the benefits of revascularization versus medical therapy in substudies of major trials (5–7). Relative perfusion quantification is most relevant as a surrogate biomarker endpoint in trials for therapies aimed at reducing myocardial ischemia. For example, one recent study evaluated the influence of angiogenic gene therapy on relative myocardial perfusion, demonstrating significant reductions in total perfusion deficit and reduction in anginal frequency (8). However, when designing such trials care must be taken to ensure the chosen biomarker does not undermine the trial results. For example, in the ISCHEMIA trial, patients with moderate to severe ischemia were randomized to early invasive versus conservative strategy. Subsequent analysis showed that ischemia at baseline was not predictive of adverse cardiovascular events (9), a finding that contradicts half a century of data. Although this observation may reflect the inclusion of only patients with moderate to severe ischemia (which minimizes the difference between patients), it raises important questions regarding the measures of ischemia. The trial relied on visual semiquantitative analysis of perfusion, which is less reproducible than quantitative analysis (10). The readers were not masked to the ischemia inclusion requirements. Additionally, the combination of ischemia estimates from different modalities may have precluded the ability to effectively select patients. These observations underscore the importance of minimizing variability when using surrogate biomarkers in clinical trials.
Absolute Myocardial Blood Flow (MBF)
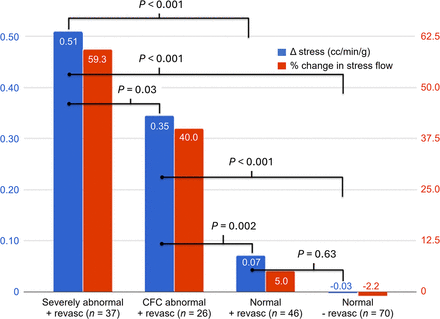
PET myocardial perfusion imaging enables accurate quantification of absolute MBF, reflecting the integrated hemodynamic effect of disease across the entire coronary circulation. Stress absolute MBF and myocardial flow reserve provide independent and incremental prognostic value over clinical risk factors and relative myocardial perfusion. Quantitative flow metrics are sensitive to changes in atherosclerosis and coronary microvascular function and have been used to test the effects of medical therapies targeting early atherosclerosis (e.g., statins) (11), microvascular disease from various etiologies (e.g., antiinflammatory therapies) (12), and cardiometabolic disease (13,14). In addition, stress MBF and myocardial flow reserve are sensitive markers of ischemia with epicardial coronary artery disease and have been used to assess therapies aimed to reduce myocardial ischemia and the efficacy of revascularization (15,16). Results from one study (17) evaluating the influence of revascularization on MBF are shown in Figure 2.
Effect of revascularization on absolute stress MBF. Severely abnormal was defined as both relative perfusion abnormality of ≥10% and ≥10% severely reduced absolute MBF as coronary flow capacity. Abnormal coronary flow capacity included patients with ≥10% severely reduced coronary flow capacity without significant relative perfusion abnormality. Study highlights potential for absolute MBF measurements to act as surrogate biomarker for intervention (revascularization) but also to select patients most likely to benefit. CFC = coronary flow capacity. (Reprinted from (17).)
Most trials incorporating perfusion or flow surrogate measures use serial imaging to compare changes over time. To minimize variability between studies, consistent protocols, stressors, hardware, and software should be used. Automated measurements may be preferable for the reasons outlined above. Despite technical advancements, reproducibility challenges persist, underscoring the need for continued improvements in standardization and methodology.
Microcalcification
Beyond evaluation of regional myocardial perfusion and absolute MBF, PET has the potential to image biologic activity of atherosclerosis. Since calcification plays a critical role in plaque development, there has been growing interest in quantifying microcalcification activity. 18F-NaF PET shows great promise for identifying “biologically active” plaques in patients at high risk for coronary events (18). Although microcalcification activity was not linked to major cardiac events (including revascularization) among patients with a recent coronary event, 18F-NaF uptake remained a predictor of cardiac death and nonfatal myocardial infarction (19). These findings support the use of plaque activity imaging for clinical trials of drugs aimed at reducing cardiovascular mortality.
Though we lack randomized trials validating coronary 18F-NaF PET-based therapy decisions, initial observational studies are encouraging. With novel lipid-lowering and antiinflammatory medications emerging, there is a need for precision medicine, with individualized patient-specific approaches. PET can help tailor these therapies to patients with active disease, potentially improving outcomes while reducing unnecessary treatments. One study demonstrated that 6 mo of rosuvastatin therapy reduced coronary 18F-NaF uptake (20), suggesting PET could not only guide therapy but also assess its effectiveness.
Fibrosis and Inflammation
Although 18F-NaF is the leading coronary plaque PET tracer with robust outcome data, other tracers, such as 18F-FDG and 68Ga-DOTATATE, have shown promise in evaluating the antiinflammatory effects of therapy at the atherosclerotic lesion level. 18F-FDG is highly reproducible (21) and has excellent test–retest reliability and low inter- and intraobserver variability (22). As a result of these strengths, it has been used as a marker of plaque activity in clinical trials. For instance, a phase 2 trial of dalcetrapib, a cholesteryl ester transfer protein inhibitor, found no effect on 18F-FDG uptake in carotid and aortic plaques (23), which was consistent with the subsequent phase 3 trial that failed to show clinical efficacy in nearly 16,000 patients with a recent acute coronary syndrome (24).
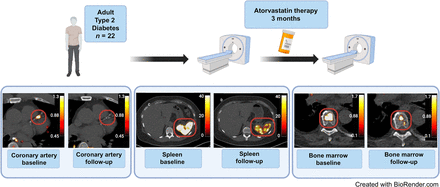
68Ga-DOTATATE has the advantage of specifically binding to somatostatin type 2 receptors, decreasing background noise from adjacent myocardial tissue. In one study (Fig. 3), coronary 68Ga-DOTATATE uptake decreased after 3 mo of atorvastatin therapy (40 mg) in patients with type 2 diabetes mellitus (25). These tracers have also demonstrated utility in diagnosing and monitoring large-vessel vasculitis, such as giant cell and Takayasu arteritis (26). These findings suggest that 18F-FDG and 68Ga-DOTATATE could serve as surrogate biomarker endpoints in trials for coronary artery disease therapies and immunosuppression, potentially providing earlier detection of therapeutic benefits.
Design of study evaluating impact of atorvastatin therapy on 68Ga-DOTATATE uptake in coronary arteries, bone marrow, and spleen. Radiotracer activity in all locations decreased after 3 mo of therapy, suggesting that 68Ga-DOTATATE may have role as surrogate biomarker of vascular and hematopoietic inflammation in clinical trials. (Reprinted from (25).)
Thrombosis
Thrombus formation plays a central role in coronary artery disease, yet noninvasive imaging of active thrombi remains challenging due to limitations in sensitivity and specificity. A novel radiotracer, 18F-labeled fiban-class ligand (GP1), which binds to the activated glycoprotein IIb/IIIa receptor on platelets, has shown promise in imaging both venous and arterial thrombi. In patients with acute myocardial infarction, 18F-GP1 accurately identified culprit plaques with 100% specificity and 80% sensitivity (molecular targeting trial; Table 1) (27). It has also been used to detect intramyocardial hemorrhage, left ventricular thrombus, and atrial appendage thrombus, offering a comprehensive view of the thrombotic causes and consequences of myocardial infarction and stroke (27). The ability to track thrombus formation with 18F-GP1 allows clinicians to define thrombotic causes, initiate appropriate therapies (such as anticoagulation), and monitor response to treatments. Unlike traditional outcome measures such as vessel patency on invasive angiography, 18F-GP1 offers a direct, quantitative method for assessing the efficacy of novel antithrombotic therapies on thrombus burden.
Valvular Heart Disease
Molecular PET imaging enables the assessment of disease activity in valvular heart disease, particularly aortic stenosis. 18F-FDG imaging can detect inflammation within the valve, which correlates with disease severity and progression (28). In aortic stenosis, 18F-NaF identifies microcalcification activity in regions that develop macroscopic valve calcification. Baseline uptake of 18F-NaF is associated with aortic stenosis progression, as measured using aortic valve calcium scoring and echocardiography (28). On this basis, 18F-NaF PET has been used as an imaging endpoint in trials assessing the ability of novel therapeutic strategies for aortic stenosis progression (29). In a randomized controlled trial, patients with aortic stenosis were randomized to denosumab, alendronic acid, or placebo and underwent serial echocardiography, CT, and 18F-NaF PET (29). Although both interventions inhibited bone turnover, neither affected valvular 18F-NaF uptake or the progression of aortic valve disease. This trial highlighted the utility of 18F-NaF PET in confirming the lack of effect on the intended disease process (29).
Beyond aortic stenosis, 18F-NaF has also been used to assess calcification activity in mitral annular calcification and bioprosthetic valve degeneration (30), where it predicts disease progression and clinical events. Further studies are needed to determine whether the 18F-NaF PET signal can be modulated by therapeutic intervention. Exploring other PET tracers in valvular heart disease, such as 68Ga-DOTATATE for inflammation and 18F-GP1 for thrombosis, may also offer valuable insights.
Amyloidosis
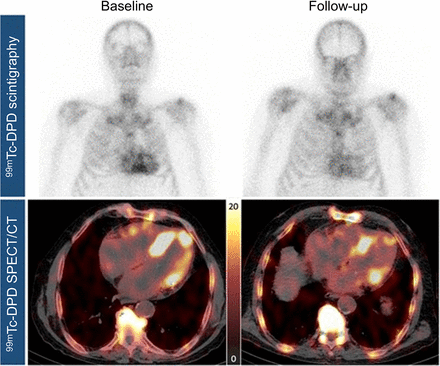
Transthyretin cardiac amyloidosis (ATTR-CM) is an increasingly recognized cause of heart failure. Cardiac SPECT imaging of bone scintigraphy radiotracers has been established as a highly accurate diagnostic tool for ATTR-CM, with PET-based radiotracers emerging as additional options (31). Although the mechanism behind bone scintigraphy radiotracer binding in ATTR-CM remains unclear (32), quantitative analysis of uptake correlates with cardiovascular MR measures of disease severity (33) and is predictive of heart failure hospitalization or cardiovascular death (34). SPECT-based quantitative measures decrease in response to medical therapy, with results from one study shown in Figure 4. However, the changes in radiotracer uptake do not correlate with functional changes (35). These results suggest that that molecular changes seen in bone scintigraphy radiotracers may either be unrelated to disease progression or precede functional improvements.
Case from study evaluating quantitative measures of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid uptake at baseline and follow-up on therapy. There were significant reductions in SUVs suggesting positive response to therapy. DPD = 3,3-diphosphono-1,2-propanodicarboxylic acid. (Reprinted from (37).)
Several PET-based radiotracers have shown specificity in binding to amyloid fibrils, making them potentially useful for tracking disease burden in patients with ATTR-CM as well as other forms of cardiac amyloidosis, such as light-chain amyloidosis (36). Multiple case series and studies have demonstrated that radiotracer uptake (using PET and SPECT agents) can decrease in patients receiving targeted therapy for ATTR-CM (37,38). For instance, Fontana et al. demonstrated that patisiran therapy in patients with hereditary ATTR-CM led to a reduction in percentage injected dose (a measure of radiotracer activity) for 3,3-diphosphono-1,2-propanodicarboxylic imaging, with similar reductions observed in extracellular volume (38).
Several ongoing trials are evaluating reductions in radiotracer uptake in response to therapy for cardiac amyloidosis. One study (NCT05635045) is assessing changes in 124I-evuzamitide PET radiotracer uptake, which binds directly to amyloid fibrils, between baseline and after 1 y of targeted therapy (surrogate biomarker endpoint trial). Trials using surrogate biomarkers are particularly important in cardiac amyloidosis due to the slow progression of the disease and high risk of noncardiac mortality, which can obscure outcomes in event-driven trials. Furthermore, the rapid expansion of potential therapies highlights the need for imaging-biomarker–guided trials to more efficiently evaluate and compare these emerging treatments.
Cardiac Sarcoidosis
Glucose Metabolism
Cardiac sarcoidosis, a manifestation of the multisystem inflammatory disease sarcoidosis, can lead to serious complications such as arrhythmias, heart failure, and sudden cardiac death. Diagnosing cardiac sarcoidosis is challenging due to its variable presentation, often requiring a combination of clinical, imaging, and histopathologic findings. Traditional endomyocardial biopsy has low sensitivity (∼25%), making noninvasive imaging crucial. 18F-FDG PET plays a central role in the diagnosis and management of cardiac sarcoidosis (31). When combined with myocardial perfusion imaging, it provides comprehensive insights into both inflammation and fibrosis. This dual approach improves diagnostic accuracy and guides treatment decisions (31).
Various quantitative PET measures, such as SUV, target-to-background ratios, and volumetric measures, are used to assess disease activity (31,39). Although used in clinical practice—especially in serial imaging, to follow the effects of immunosuppressive therapy—SUV-based measurements are prone to variability due to patient preparation, technical factors, and imaging protocols and have not been consistently linked to clinical outcomes.
Inflammatory Cell–Directed Radiotracers
In addition to 18F-FDG, several novel PET tracers have been studied for cardiac sarcoidosis. 68Ga-DOTATATE, which targets somatostatin receptors, has shown promise in detecting granulomatous inflammation (40), offering an alternative to 18F-FDG–based imaging. Other tracers under investigation include 11C-methionine (41), which detects amino acid metabolism in inflammatory cells, and 68Ga-pentixafor (42), which has high affinity for chemokine receptor 4, known for its increased expression in areas of inflammation. However, most of these tracers have been studied only in small populations, and their clinical utility in larger cohorts, especially as surrogate biomarkers, remains to be fully established. Nevertheless, these novel tracers hold potential for providing improved specificity in detecting inflammation and may offer additional molecular targets for monitoring disease activity and response to therapy in cardiac sarcoidosis (31).
The CHASM trial (NCT03593759), the only randomized study to date to address sarcoidosis treatment, focuses on the optimal initial treatment strategy for patients with active disease (43). Patients are randomized to receive different immunosuppressive therapies, with relative myocardial perfusion as the primary endpoint (surrogate biomarker endpoint trial) and 18F-FDG findings as secondary endpoints (43). Imaging biomarkers in this trial are being used to provide critical insights into disease activity, helping to evaluate the effectiveness of different treatments and refine therapeutic strategies for cardiac sarcoidosis.
Heart Failure
Beyond assessing ischemic cardiomyopathy with myocardial perfusion imaging, nuclear cardiology offers approaches to evaluating arrhythmia risk in heart failure patients through imaging of the autonomic nervous system using 123I-meta-iodobenzylguanidine (MIBG). 123I-MIBG imaging visualizes abnormalities in cardiac sympathetic innervation, A reduced heart-to-mediastinum ratio has been linked to increased arrhythmia risk (44). The AdreView Myocardial Imaging for Risk Evaluation in Heart Failure study used 123I-MIBG imaging as a surrogate endpoint to determine the risk of heart failure progression and sudden cardiac death (45), finding that a heart-to-mediastinum ratio of less than 1.6 was an independent predictor of arrhythmia risk (46).
Small, randomized studies have demonstrated that heart failure therapies, including angiotensin-converting enzyme inhibitors (47) and β-blockers, can increase the heart-to-mediastinum ratio (48). However, despite these findings, 123I-MIBG has not translated into changes in clinical practice or as a surrogate biomarker in trials. This is partly due to its unclear relationship with specific therapies and the high event rates in heart failure patients, which limit the benefit of using 123I-MIBG as a surrogate biomarker.
Cardiac Transplant
In patients who survive the first year after cardiac transplantation, cardiac allograft vasculopathy is a leading cause of death (49). Therefore, monitoring for cardiac allograft vasculopathy in these high-risk population is critical. Studies have demonstrated that MBF is predictive of cardiac allograft vasculopathy and cardiovascular events (50), and changes in MBF mirror the changes in coronary intimal thickening (51). One ongoing study (NCT06089486) (diagnostic trial) is comparing surveillance strategies of myocardial perfusion imaging (PET MBF) with invasive coronary imaging.
REGULATORY CONSIDERATIONS
To use an imaging biomarker as an endpoint, requirements from the Food and Drug Administration need to be met. Biomarkers undergo a formal regulatory review process directed by the 21st Century Cures Act. The initial letter of intent includes information on the context of use, which includes the biomarker category as well as the intended use (52). Biomarkers are categorized according to the Biomarkers, EndpointS, and other Tools (BEST) categorization. The BEST categories most relevant for surrogate biomarker trials include diagnostic, monitoring (e.g., to detect a change in the extent of disease), predictive (e.g., identifying individuals most likely to benefit), and pharmacodynamic response (e.g., efficacy biomarkers) (53). Monitoring biomarkers are potentially relevant in molecular targeting trials or as a surrogate endpoint; predictive biomarkers could be used to enrich patient populations in clinical trials; and pharmacodynamic response biomarkers could be used as a surrogate endpoint. Once the context of use has been established, a qualification plan is developed that summarizes the existing evidence, knowledge gaps, and plan for addressing those gaps (52). The Food and Drug Administration must ultimately review and approve a full qualification application, which includes all information regarding methodology and performance characteristics for the biomarker (52). Importantly, when evidence is presented to the Food and Drug Administration, participants in clinical trials should be representative of the patients who will use the medical products. This is specified in a Diversity Action Plan including the age group, ethnicity, sex, and race of clinically relevant study populations.
CHALLENGES AND LIMITATIONS
Although imaging surrogate biomarkers hold great promise, several key challenges must be addressed for their effective application.
Need for Reproducibility
A successful biomarker must be reproducible. One challenge is the intrinsic physiologic variability, which can be minimized by close alignment with validated standards and adhering to standardized protocols and quantitative analysis. However, these stringent requirements could potentially limit the number of sites able to participate in multicenter studies. Although nuclear cardiology is inherently quantitative, measurements are often derived by subjective visual placement of regions of interest, increasing the overall test variability. However, the reproducibility of quantitative measures can be improved through automated approaches. For example, quantitative analysis of perfusion is more consistent than expert visual interpretation (10,14). By detecting small but clinically meaningful differences in surrogate biomarkers, quantitative analysis enhances the ability to predict therapy responses. Automating quantification is crucial for biomarkers that currently rely on manual segmentation. Recent studies have evaluated deep learning–based approaches for fully automated segmentation and quantitation of SPECT/CT pyrophosphate imaging (34), an approach that could be extended to any hybrid imaging study. Ensuring consistent quantification is particularly important when tracking changes in quantitative measures over time. In the context of clinical trials, reproducibility is essential to validate biomarkers as reliable endpoints. Automated, quantitative methods can provide the consistency needed to reduce interoperator variability and improve the reliability of trial results, facilitating regulatory approval and adoption in clinical practice.
Need for Mechanistic Link
A biomarker’s utility hinges on a clear mechanistic link to the underlying disease process. This connection must be thoroughly understood and well delineated, as it forms the foundation for interpreting biomarker changes in relation to disease progression or treatment response. Without a solid understanding of the pathophysiologic pathway, the biomarker’s relevance becomes questionable, limiting its potential to be effectively translated into clinical research or practice. Furthermore, regulatory agencies and clinical trial frameworks increasingly demand strong mechanistic evidence to validate biomarkers as reliable indicators of disease or therapeutic efficacy. Establishing this link is critical for scientific rigor and for ensuring the biomarker’s clinical applicability and acceptance. Further studies strengthening the mechanistic understanding of the molecular targets are essential to aid clinical trials.
Need for Data Diversity
It is essential that the population in which the biomarker will be applied is comparable to the populations in which it was validated. Clinical trials have systematically enrolled fewer female and racial/ethnic minority patients, and similarly poor underrepresentation is seen in many observational studies that help identify potential surrogate biomarkers. For example, despite the genetic predisposition of Black patients to ATTR-CM, most screening tools have been developed and validated in predominantly White cohorts (54). Furthermore, studies have reported significant racial and ethnic differences in biomarker expression and response. Hackler et al. found that Black individuals had higher lipoprotein(a), leptin, D-dimer, high-sensitivity C-reactive protein, and NT-proBNP which may contribute to racial differences in the development and complications of CVD (55). Therefore, before using an imaging biomarker in clinical trials it is critical to understand the racial, ethnic, and sex-based variations.
FUTURE BIOMARKERS
There are many potential radiotracers that could play an important role as surrogate biomarkers. One such novel radiotracer, 68Ga-fibroblast activation protein inhibitor (FAPI), binds exclusively to activated fibroblasts. Activated fibroblasts are the key cells driving cardiomyopathic disease and are therefore an important treatment target. Increased 68Ga-FAPI signal has been observed across a range of heart muscle conditions, including myocardial infarction, hypertrophic cardiomyopathy, and cardiac sarcoidosis (56). 68Ga-FAPI uptake correlates with areas of myocardial fibrosis in patients with systemic sclerosis (57) and correlates with measures of myocardial stress in patients with severe aortic stenosis (58). In patients with acute myocardial infarction, intense 68Ga-FAPI can be observed in the acute infarct zone as well as the periinfarct zone extending beyond the infarct defined by late gadolinium enhancement (59). Further studies are required to assess whether baseline imaging predicts disease progression and whether changes in signal are observed with novel therapeutic interventions. There are several other potential surrogate biomarkers that may become relevant in the future, particularly if theranostic pairs can be derived successfully using nanoparticles or other techniques.
CONCLUSION
Nuclear cardiology imaging surrogate biomarkers have already carved out a vital role in managing several cardiovascular conditions and hold immense potential for broader application. These biomarkers accelerate the development and evaluation of cardiovascular therapies, offering a faster path from discovery to clinical practice. However, the future lies in refining the link between imaging outcomes and real-world patient results, understanding of mechanistic links, advancing quantitative techniques, and ensuring robust validation across diverse populations, especially underrepresented minorities. As research progresses, these efforts will strengthen the acceptance of surrogate biomarkers as endpoints in clinical trials, ultimately propelling cardiology forward at a faster and more dynamic pace.
DISCLOSURE
Robert Miller received consulting fees from BMS and Pfizer and research support from Pfizer and Alberta Innovates. Piotr Slomka participates in software royalties for QPS software at Cedars-Sinai Medical Center and has received research grant support from Siemens Medical Systems and consulting fees from Synektik, SA. Krishna Patel reports an institutional research grant from Jubilant DraxImage. Leandro Slipczuk reports institutional grants from Amgen and Philips. David Newby has received reagents for the synthesis of radiotracers from Life Molecular Imaging and SOFIE. Panithaya Chareonthaitawee receives/received consulting fees from Clario and GE HealthCare and royalties from UpToDate. No other potential conflict of interest relevant to this article was reported.
- © 2025 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication June 25, 2024.
- Accepted for publication December 5, 2024.