Abstract
Whereas benign pheochromocytomas and paragangliomas are often successfully cured by surgical resection, treatment of metastatic disease can be challenging in terms of both disease control and symptom control. Fortunately, several options are available, including chemotherapy, radiation therapy, and surgical debulking. Radiolabeled metaiodobenzylguanidine (MIBG) and somatostatin receptor imaging have laid the groundwork for use of these radiopharmaceuticals as theranostic agents. 131I-MIBG therapy of neuroendocrine tumors has a long history, and the recent approval of high-specific-activity 131I-MIBG for metastatic or inoperable pheochromocytoma or paraganglioma by the U.S. Food and Drug Administration has resulted in general availability of, and renewed interest in, this treatment. Although reports of peptide receptor radionuclide therapy of pheochromocytoma and paraganglioma with 90Y- or 177Lu-DOTA conjugated somatostatin analogs have appeared in the literature, the approval of 177Lu-DOTATATE in the United States and Europe, together with National Comprehensive Cancer Network guidelines suggesting its use in patients with metastatic or inoperable pheochromocytoma and paraganglioma, has resulted in renewed interest. These agents have shown evidence of efficacy as palliative treatments in patients with metastatic or inoperable pheochromocytoma or paraganglioma. In this continuing medical education article, we discuss the therapy of pheochromocytoma and paraganglioma with 131I-MIBG and 90Y- or 177Lu-DOTA-somatostatin analogs.
Pheochromocytomas and paragangliomas are rare neuroendocrine tumors (NETs) typically arising in chromaffin tissue, with an overall incidence of 0.4–2.1 cases per million people (1), although higher incidences may be seen in pathology series. In accordance with World Health Organization guidelines, these tumors are classified as paragangliomas (2); we will refer to them as pheochromocytomas when they arise from the adrenal medulla and as paragangliomas when extraadrenal. Pheochromocytoma and paraganglioma most frequently arise from sympathetic tissues. Those arising from parasympathetic tissue are most often in the head and neck. In our previous article, we reviewed the genetics, phenotype, presentation, and imaging characteristics of pheochromocytoma and paraganglioma (3).
Patients with pheochromocytoma and paraganglioma often present with symptoms of elevated catecholamines, depending on the genotype. Tumors of sympathetic origin often secrete elevated levels of epinephrine or norepinephrine or of their metabolites metanephrine and normetanephrine. Tumors of parasympathetic origin may secrete elevated levels of dopamine or its metabolite 3-methoxy-tyramine but are often asymptomatic. A small percentage of pheochromocytoma and paraganglioma are biochemically silent.
Although only 2%–26% of pheochromocytomas and paragangliomas are metastatic (4), benign and malignant tumors cannot be differentiated histologically; thus, the term metastatic rather than malignant is used when tumor is found outside the normal sites of chromaffin or paraganglia tissues (2). Common sites of metastatic disease include the lymph nodes, bone, liver, and lung (4). Currently, systemic radiopharmaceutical therapies (RPT) for pheochromocytoma and paraganglioma include 131I-metaiodobenzylguanidine (iobenguane, 131I-MIBG) of low specific activity (LSA) or high specific activity (HSA) and peptide receptor radionuclide therapy (PRRT) with 90Y- or 177Lu-DOTA-somatostatin analogs.
GENERAL CONSIDERATIONS FOR SYSTEMIC RPT IN PHEOCHROMOCYTOMA AND PARAGANGLIOMA
Although most patients with benign pheochromocytoma and paraganglioma are cured with surgical resection, and local control of head and neck pheochromocytoma and paraganglioma is usually achieved with definitive external-beam radiotherapy, approximately one third are not cured and require long-term follow-up. Patients with benign disease have a survival rate similar to that of healthy individuals, whereas patients with metastatic disease have a 5-y survival rate of 55%–92% (4,5). The wide differences in survival of pheochromocytoma and paraganglioma patients are related to several variables, including genetic status, size of primary tumor, biochemical phenotype, and presence of metastatic disease at initial diagnosis (6,7). Long-term stability over 15 y is not uncommon. Since current treatments of metastatic disease are rarely curative, a watch-and-wait approach is often appropriate, reserving therapy for progressive or symptomatic disease that is not responsive to medical treatment or amenable to surgical resection (8). Systemic treatment is also an option for inoperable nonmetastatic disease that is progressing or has refractory symptoms.
The goals of treatment are palliative, including prevention of progression and improvement of symptoms related to tumor burden or catecholamine release. For systemic treatment, the most common chemotherapy regimen is cisplatin, vinblastine, and dacarbazine, which may be preferred for rapidly progressing disease (9). Tyrosine kinase inhibitors that target the vascular endothelial growth factor pathway, such as sunitinib, may have efficacy (10). External radiation for localized symptomatic disease is generally successful for local control (11). For less urgent treatment, RPT with LSA or HSA 131I-MIBG and PRRT are available. Except for HSA 131I-MIBG, none of these approaches are currently approved by the U.S. Food and Drug Administration (FDA) for pheochromocytoma and paraganglioma.
PATIENT PREPARATION BEFORE RPT
When catecholamines or their metabolites (metanephrines) are elevated, experts recommend catecholamine blockade before invasive intervention or RPT; the target is blood pressure control, but often labile hypertension persists (12). Typically, first-line treatment consists of α-blockade (e.g., phenoxybenzamine or doxazocin). A concomitant β-blocker (e.g., atenolol or metoprolol) is often required but should never be used without adequate α-blockade, because unopposed α-adrenergic receptor stimulation could precipitate a hypertensive crisis. Occasionally, addition of metyrosine is needed to block catecholamine synthesis.
Catecholamine release symptoms (hypertension, tachycardia, palpitation, headache, chest pain, tachypnea, sweating, cutaneous flushing, anxiety, and the more severe or life-threatening symptoms of stroke and myocardial infarction) (13,14) are possible during PRRT and MIBG infusion or in the early posttreatment period (13,15). Gonias et al. (15) reported acute hypertension in approximately 14% of subjects and in 15% of high-radioactivity LSA 131I-MIBG treatments, occurring approximately 30 min after initiation of infusion and requiring treatment. Sometimes hypertension recurred on repeat treatment. Although it is difficult to predict who will develop a hypertensive response to RPT, patients who are biochemically silent will not. Although no acute hypertensive crises were observed with HSA 131I-MIBG, the package insert indicates an 11% incidence of grade 3 or 4 hypertension within 24 h of infusion. Physicians administering RPT should be prepared to treat blood pressure elevation with oral or parenteral antihypertensives (e.g., phentolamine). Close coordination with other medical specialties, including intensive care, is prudent.
Because of the teratogenic effects of ionizing radiation, patients receiving RPT should avoid conception (7 mo for women and 4 mo for men) and must not be pregnant or breastfeeding during treatment. Patients must also be willing and able to follow radiation safety precautions.
POST-RPT RADIATION PROTECTION
In the United States, the Nuclear Regulatory Commission regulations allow for discharge of patients receiving RPT if exposure to others is not likely to exceed 0.5 cGy, and written instructions must be provided to outpatients if the dose is likely to be more than 0.1 cGy. Other countries require RPT to be administered as an inpatient procedure or use different activity levels as the cutoff. Even if release criteria for outpatient administration are met, inpatient treatment may be prudent for some patients. In the United States, outpatient therapies are often performed when LSA 131I-MIBG therapy is less than 9.25 GBq or no more than 7.4 GBq of 177Lu-DOTATATE, whereas with the recommended dose of approximately 18.5 GBq of HSA 131I-MIBG, treatment must be inpatient.
131I-MIBG THERAPY
131I-MIBG was first described by Wieland et al. for imaging the adrenal medulla (16). At present, 123I-MIBG is preferred for imaging and 131I-MIBG for therapy. Currently, 131I-MIBG is seldom used for imaging pheochromocytoma and paraganglioma except for performing dosimetry and for documenting posttreatment targeting. The physical properties of 131I-MIBG make it a useful therapeutic agent, including a short-range 606-keV β-emission and a 364-keV γ-ray that allows imaging and dosimetry. Furthermore, its 8.02-d half-life allows flexibility for labeling and shipping.
MIBG is a derivative of guanethidine and a substrate for the norepinephrine (noradrenaline) transporter. Many pheochromocytomas and paragangliomas express norepinephrine (noradrenaline) transporter, as do other NETs (17). Most reports of 131I-MIBG therapy in patients with pheochromocytoma and paraganglioma are retrospective, small, and use LSA 131I-MIBG (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). No direct therapeutic comparison studies of LSA 131I-MIBG and HSA 131I-MIBG have been performed.
131I-MIBG Production
LSA 131I-MIBG preparations (0.555–1.85 MBq/mg) typically contain approximately 1/2,000 MIBG molecules labeled with 131I. Because uptake via norepinephrine (noradrenaline) transporter is saturable, a high administered mass of unlabeled MIBG could competitively inhibit uptake of the 131I-MIBG, potentially reducing therapeutic efficacy, as has been demonstrated in vitro and in preclinical studies (18). Additionally, because MIBG is a competitive inhibitor of catecholamine reuptake, a high administered mass of MIBG can cause pharmacologic effects (19). This is rarely an issue for diagnostic administrations (low mass) but occurs more frequently during therapeutic administration of LSA 131I-MIBG (high mass).
In contrast, most MIBG molecules are labeled with 131I in preparations of HSA 131I-MIBG (92.5 GBq/mg). HSA 131I-MIBG (Azedra; Progenics Pharmaceuticals, Inc.) was the first radiopharmaceutical the FDA approved to treat metastatic or inoperable pheochromocytoma or paraganglioma (20,21). Use of HSA 131I-MIBG reduces the risk of infusion reactions and may enhance the therapeutic ratio (19,21,22), although the latter has not yet been proven in patients (23).
Patient Selection and Preparation for 131I-MIBG RPT
Published procedure guidelines for 131I-MIBG therapy preceded availability of HSA 131I-MIBG. Nonetheless, these guidelines are broadly applicable to HSA 131I-MIBG (24). When MIBG therapy is being considered, a positive 123I-MIBG scan is required, as only patients with MIBG-avid disease are candidates for therapy. In our opinion, a 123I-MIBG scan performed within 3–6 mo is adequate if there is no intervening therapy, especially if a 131I-MIBG dosimetric scan will be performed. The amount of uptake required to allow therapy to be considered is poorly defined. Criteria have included visual assessment (uptake clearly visible above the background level), semiquantitative assessment (lesion–to–background uptake > 2), and estimates of tumor dose or more than 1% injected dose in tumor (17). Because tumors can lose MIBG avidity over time or after treatment, confirmation of avidity around the time of treatment is important, especially if the patient’s symptoms have recently changed. Patients must be screened for marrow function (minimum platelets, 80,000/μL; minimum neutrophils, 1,200/μL) and renal function (minimum creatinine clearance, 30 mL/min). To protect the thyroid by preventing uptake of free 131I, stable iodine must be administered unless the thyroid is absent or previously ablated. Typically, 130 mg of an oral solution of potassium iodide (SSKI; Avondale Pharmaceuticals) is administered 24–48 h before therapy and continued for 10–15 d (24).
131I-MIBG Administration
HSA 131I-MIBG
Since the approval of HSA 131I-MIBG by the FDA, there has been a surge of interest in MIBG therapy. The recommended dose is 296 MBq/kg in patients weighing no more than 62.5 kg or 18,500 GBq in those over 62.5 kg, given twice at least 90 d apart (20). With these doses, virtually all patients exceed Nuclear Regulatory Commission limits for outpatient treatment. Inpatients must be confined to rooms with appropriate shielding, and preparations must be made to limit exposure of other people to the radiation (in the United States, this is usually for 3 nights).
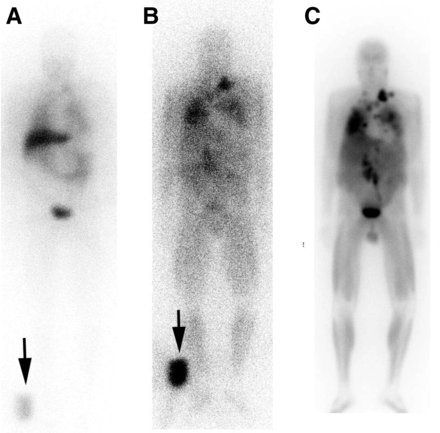
At dosimetric doses of less than 222 MBq, HSA 131I-MIBG can be injected over 1 min, whereas therapeutic doses are administered over 30 min in adults and 60 min in children. Representative images for dosimetry and posttreatment HSA 131I-MIBG are shown in Figure 1. Occasionally, there is a transient burning pain in the infusion vein, resolving almost immediately with saline flushing.
Anterior HSA 131I-MIBG images in 60-y-old man with metastatic paraganglioma. (A and B) Dosimetric images were performed 1 h (A) and 96 h (B) after intravenous administration of 185 MBq of HSA 131I-MIBG with standard in field of view (arrows) to allow calculation of organ dose limits. Images show multiple metastases that increase in contrast over time. (C) Three days after therapy with 18.4 GBq of HSA 131I-MIBG, imaging revealed robust retention in sites of disease, including some not visible on dosimetric images. Patient exhibited markedly reduced hormonal symptoms, including decreased need for antihypertensive medications lasting over 2 y.
LSA 131I-MIBG
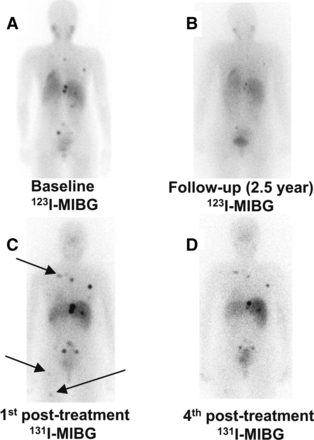
In contrast to the 2-injection regimen recommended for HSA 131I-MIBG, various approaches using different activities and cycles of LSA 131I-MIBG have been reported (Supplemental Table 1) (17). Most inject approximately 7.4 GBq (25–28); 1 group injected approximately 18.5 GBq (29,30). The University of California San Francisco group has used a much higher activity, up to 666 MBq/kg, in conjunction with stem cell support. In the only direct comparison of a very low activity (5.55 GBq) versus a low-to-intermediate activity (9.25–12.95 GBq), the low-to-intermediate activity had a more rapid onset of efficacy at the expense of increased acute and chronic toxicity (31). Representative diagnostic 123I-MIBG images and post–LSA 131I-MIBG images are shown in Figure 2. Others have suggested an improved response with higher single administered injections of more than 18.5 GBq versus less than 18.5 GBq (29).
Patient with metastatic pheochromocytoma to bone with bone pain. Patient received 4 treatments of LSA 131I-MIBG (111 MBq/kg; range, 5.74–6.18 GBq per treatment) totaling 24.01 GBq over 14 mo. Patient showed symptomatic improvement in bone pain. 123I-MIBG scans were obtained at baseline (A) and at 2.5 y (B) after last 131I-MIBG treatment. (C) 131I-MIBG posttherapy scan a few days after first LSA 131I-MIBG treatment shows better visualization of lesion, and more lesions are evident (arrows) than on baseline 123I-MIBG scan (A). (D) 123I-MIBG scan after all treatments (B) and last 131I-MIBG posttreatment scan (D) shows decreased uptake in bone lesions, probably related to treatment. (Reprinted with permission of (66).)
Although high-activity therapies of up to 666 MBq/kg are common in children with neuroblastoma in conjunction with autologous stem cell support, an at least 444 MBq/kg activity of LSA 131I-MIBG in adults with pheochromocytoma or paraganglioma appears to have high rates of toxicity, particularly pulmonary (15). Therefore, doses higher than 296 MBq/kg are not recommended.
In contrast to HSA 131I-MIBG treatment (∼18.5 GBq), low-activity treatments (≤9.25 GBq) can be administered to most patients as outpatients. Therefore, in patients who have relatively indolent disease or who are unwilling to undergo inpatient therapy, serial low-dose treatments can be considered. The most common approaches to low-dose therapy are 74–111 MBq/kg or 7.40 GBq/cycle administered 3 mo apart. Many practitioners give 3–4 cycles of therapy followed by reassessment and consideration of further therapy.
Because LSA 131I-MIBG treatments contain a much higher mass of MIBG than do HSA 131I-MIBG treatments, the former are usually administered over 1–2 h (15,17,25,27,32), although shorter infusions have been performed (26,30). Unlike HSA 131I-MIBG, which is not associated with hypertension during infusion, hypertension occurs during 6%–14% of LSA 131I-MIBG infusions, sometimes requiring pausing or decreasing the infusion rate and using antihypertensive treatment (15,21,33,34). Nonetheless, significant worsening of preexisting hypertension in the first 24 h is reported in 11% of patients receiving HSA 131I-MIBG and may also occur with LSA 131I-MIBG.
Clinical Experience with 131I-MIBG in Pheochromocytoma and Paraganglioma
There have been few controlled prospective trials and no phase III clinical trials of 131I MIBG therapy. Loh et al. reviewed the experience of 21 centers using LSA 131I-MIBG receiving an average of 3 (range, 1–11) single injections of 3.55–11.1 GBq and cumulative activities of 3.55–85.91 GBq (35). Thirty percent of patients had at least partial responses, and 4% had complete responses. Furthermore, there was at least a partial hormonal response in 45% of patients, 13% of whom had a complete response. A metanalysis that included 243 patients with metastatic pheochromocytoma or paraganglioma receiving median cumulative activities ranging from 6.88–39.4 GBq with a median of 1–7 infusions revealed similar findings (36). Supplemental Table 1 shows therapeutic reports with 20 or more patients, including 2 reports using HSA 131I-MIBG.
The pivotal prospective phase II trial supporting FDA approval of HSA 131I-MIBG (21) enrolled 68 patients with advanced pheochromocytoma or paraganglioma and disease-related hypertension. For LSA 131I-MIBG, the largest prospective trial on pheochromocytoma and paraganglioma enrolled 50 subjects treated with varying activities of LSA 131I-MIBG, including some who received myeloablative doses and stem cell support (15). Using RECIST, both trials found a high rate of partial or durable stable disease in 92% of patients. However, an objective tumor shrinkage meeting RECIST for partial response was seen in only 22%–23% of patients. Furthermore, the peak anatomic response was often delayed, taking up to 12 mo from the first treatment. Other studies have shown somewhat higher objective response rates (34%–38%) using single administrations of approximately 14.467–18.5 GBq (29,30). At lower injected activities, objective response rates of 25%–47% were observed (26,27,31,37). In general, studies using lower single administered activities usually included a larger number of treatment cycles.
Biochemically, 31%–35% of the HSA 131I-MIBG group had a response in norepinephrine and normetanephrine levels, and 68% of patients had a reduced chromogranin A level. In comparison, therapy with LSA 131I-MIBG resulted in a complete or partial catecholamine or metanephrine response in 19%–100% of patients (Supplemental Table 1).
The endpoint of the HSA 131I-MIBG study by Pryma et al. was blood pressure control; 68% of subjects had at least a 50% reduction in antihypertensive medication use lasting at least 6 mo (38). Although no studies of LSA 131I-MIBG focused on hypertension control, a report by Thorpe et al. described improvement in 14% of hypertensive patients (30). Response in terms of overall survival and progression-free survival (Supplemental Table 1) has been variable, with some reporting progression-free survival of at least 2 y (25,30) and median overall survival of more than 3 y (21,29,30,39).
In patients with soft-tissue disease, the response to RPT is typically evaluated using anatomic imaging, most commonly CT scans. Other investigators have used functional imaging, including 123I-MIBG, but given the quantitative nature of PET in patients with 18F-FDG–avid disease, 18F-FDG has also been used for response evaluation.
Dosimetric Scans
The HSA 131I-MIBG regimen incorporates an initial dosimetric study using HSA 131I-MIBG (3.7 MBq/kg, not to exceed 222 MBq), with whole-body imaging on the day of injection, 1–2 d afterward, and 2–5 d afterward, using the MIRD schema to ensure that delivered doses are within specified limits. Usually, the dose-limiting factor is renal exposure; any required activity reduction is split equally across the 2 planned treatments.
Side Effects and Toxicity Profile
Toxicity with 131I-MIBG therapy is common; most patients recover with conservative care. The most common adverse reactions with HSA 131I-MIBG are hematologic, with grade 3–4 hematologic toxicity in about 40% of patients treated with 296 MBq/kg, increasing to over 80% at higher doses (∼444 MBq/kg). At the lower dose, 25% of patients required some hematologic support (most commonly packed red blood cells); careful monitoring of hematologic parameters after therapy is critical. Most patients reach nadir 4–6 wk after therapy, but monitoring should start no later than 2 wk after therapy. The risk of dose-limiting toxicity increases with retreatment, but because efficacy appears to remain strong, particularly in those with favorable prior responses, patients with an adequate marrow reserve can safely be treated again. The incidence of myelodysplasia and leukemia after HSA 131I-MIBG is 6.8%, similar to the 3.9%–7% reported with LSA 131I-MIBG (15,31).
A 6.8% rate of hypothyroidism is noted with HSA 131I-MIBG, and 11%–20% with LSA 131I-MIBG (25,39,40), despite attempts at thyroid blockade. Gastrointestinal toxicity is common, with nausea and vomiting in 50%–75% of patients treated with a 296 MBq/kg dose of HSA 131I-MIBG; results are anecdotally similar with LSA 131I-MIBG. However, because grade 3 or greater gastrointestinal toxicity is extremely rare, conservative care with antiemetics and hydration is almost always adequate.
Most adverse effects were seen in heavily pretreated patients. The toxicity profile in less heavily pretreated patients remains to be seen.
In general, trials describing low-dose therapy (e.g., 74 MBq/kg) have similar but less severe toxicity, with lower (but nonzero) rates of grade 3–4 toxicity. However, hematologic toxicity remains common, particularly in later cycles of therapy, and careful monitoring is still indicated.
Summary
MIBG scintigraphy is the first step to determining eligibility for 131I-MIBG therapy. Given the overlapping therapeutic responses of LSA 131I-MIBG and HSA 131I-MIBG, and that only HSA 131I-MIBG is FDA-approved, HSA 131I-MIBG is recommended in the United States. Nonetheless, considering other factors such as the cost and whether the therapy will be inpatient or outpatient, low-activity treatment strategies may be preferred, especially outside the United States. Future studies are needed to guide clinicians on choosing the MIBG treatment regimen most suited to their patients’ needs, including whether administration of a low activity and more frequent cycles of treatment with HSA 131I-MIBG would be useful. These trials should consider not only efficacy but also other parameters such as toxicity, side effects, and patient preferences.
PRRT WITH RADIOLABELED SOMATOSTATIN ANALOGS
Somatostatin receptors are highly expressed in NETs and have been targets for imaging and therapy, as reviewed recently in our continuing education article on imaging pheochromocytoma and paraganglioma (3). Somatostatin is a natural 14-amino-acid peptide hormone with regulatory effects in the endocrine system via binding to somatostatin receptors 1–5, which are highly expressed in NETs. Several somatostatin receptor agonists have been developed for imaging, and 177Lu-DOTATATE is now available for therapeutic purposes. At present, this is the only PRRT agent approved by the FDA and the European Medicines Agency. The North American Neuroendocrine Tumor Society and Society of Nuclear Medicine and Molecular Imaging jointly published guidelines addressing screening, preparation, administration, radiation safety, adverse events monitoring, and follow-up for 177Lu-DOTATATE treatment (41). Although pheochromocytoma or paraganglioma is not an approved indication for 177Lu-DOTATATE, National Comprehensive Cancer Network guidelines provide a rationale for its off-label use (42).
Patient Selection and Preparation
A prerequisite for PRRT therapy of pheochromocytoma and paraganglioma is tumor avidity on somatostatin receptor imaging. This avidity was initially determined with 111In-pentetreotide using a qualitative visual measure of uptake known as the Krenning score (43). The Krenning score uses a 4-point scale in which grade 1 indicates uptake less than in the liver; grade 2, uptake equal to that in the liver; grade 3, uptake greater than in the liver; and grade 4, uptake greater than in the spleen or kidneys. Generally, tumors should have a score of grade 2 or higher to be eligible for PRRT. Almost universally, tumors appear more prominent on 68Ga-DOTATATE PET (43); nonetheless, the same scoring concept is used, although significant differences from 111In-pentetreotide scans can be present.
Because the dose-limiting organs in PRRT are the kidneys (44), it is desirable to have normal renal function (glomerular filtration rate > 50 mL/h) before treatment. Furthermore, adequate liver and bone marrow function should be documented, with the typical parameters being a hemoglobin level higher than 8 g/dL, a white blood cell count higher than 2.0 K/μL, a platelet count higher than 70 K/μL, and a total bilirubin level lower than 3 times the upper limit of normal (41).
Most guidelines recommend stopping short- and long-acting octreotide for at least 24 h and 3–4 wk, respectively, before 90Y- or 177Lu-DOTA-somatostatin analog administration because of the risk of competition for, or blocking of, uptake. However, recent studies indicate that octreotide administration does not decrease tumor accumulation of 68Ga-DOTATATE, although decreases in normal spleen, liver, and thyroid uptake were observed (45,46).
PRRT Administration
Early 90Y-DOTATOC studies showed cases of acute renal failure related to reabsorption and retention of the agent by the proximal renal tubules (44). However, administration of amino acid solutions containing lysine and arginine blocked renal uptake by approximately 40% (47,48). Although initial protocols used commercially available amino acid mixtures such as Clinisol (Baxter International Inc.) and Aminosyn II (Abbott Laboratories), solutions containing only lysine and arginine (18–25 g total of each, 1–2 L, osmolality ≤ 1,050 mOsmol) minimize nausea and vomiting and are now preferred (41). Patients are pretreated with antiemetics followed by a continuous infusion of amino acids starting 30 min before 177Lu-DOTATATE and continued during and after treatment for a total of approximately 4 h until the entire solution has been infused.
Measured exposure rates from patients treated with 7.4 GBq of 177Lu-DOTATATE are low (under 4 mR/h at 1 min), resulting in doses to the public and caregivers of less than 0.5 cGy per year, thus allowing for outpatient treatment in the United States (49).
Clinical Experience with PRRT in Pheochromocytoma and Paraganglioma
90Y-DOTATOC and 90Y-DOTATATE are not commercially available, and their use is limited to certain academic centers. In contrast, 177Lu-DOTATATE has been approved as safe and effective in gastroenteropancreatic NETs. In pheochromocytoma and paraganglioma, small studies have also shown benefit with limited toxicity.
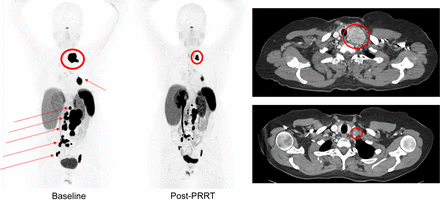
Supplemental Table 2 shows studies containing 5 or more patients receiving a 90Y- or 177Lu-DOTA-somatostatin analog for metastatic or inoperable pheochromocytoma or paraganglioma. A metaanalysis found similar response rates between 90Y-DOTATOC and 177Lu-DOTATATE, with 25% of patients demonstrating an objective response; 61%, a clinical response; and 84%, biochemical improvement. Estimated overall survival was 54.5 mo, and mean progression-free survival was 37.1 mo (50). Reports using 177Lu-DOTATATE have demonstrated significant partial regressions and stable disease as determined by anatomic imaging, as well as significant biochemical and symptomatic responses (Supplemental Table 2). Representative examples of pre- and post-PRRT 68Ga-DOTATATE and CT images are shown in Figure 3.
A 53-y-old woman with metastatic hormone-secreting SDHB-associated pheochromocytoma. Maximal-intensity-projection images of 68Ga-DOTATATE at baseline (left) vs. 12 mo after PRRT with 7.4 GBq of 177Lu-DOTATATE 4 times (middle) demonstrate significant decrease in tracer uptake in tumors in neck (encircled), left hilum, retroperitoneum, and pelvis (arrows). Baseline axial CT image (top right) through neck demonstrates large left supraclavicular mass at baseline (encircled), and post-PRRT axial CT image (bottom right) shows significant decrease in tumor size (encircled).
Dosimetry
The target organs for PRRT toxicity are the kidneys and bone marrow, as has been defined for nonpheochromocytoma and nonparaganglioma NETs. Bone marrow toxicity with a 50% drop in platelet counts is associated with a marrow dose of 2 Gy (51,52). Although a limit of 23-Gy cumulative absorbed dose can be used to avoid toxicity to the kidneys with biologically effective dose determinations, these limits could be better defined (52). Furthermore, other defined renal limits for patients with risk factors (28 Gy) may be lower than for those without risk factors (40 Gy) (53).
At present, we recommend that 7.4 GBq every 8 wk for 4 cycles, as approved by the FDA for gastroenteropancreatic NETs, also be used in patients with metastatic or inoperable pheochromocytoma or paraganglioma. Although there are no requirements for dosimetry, it should be considered if higher activities are administered. Groups that treat with more than 7.4 GBq/cycle or more than 4 cycles use dosimetry to limit the radiation dose to 23 Gy to the kidneys and less than 2 Gy to the bone marrow and have been able to administer up to 11 cycles with a maximum cumulative activity of 81.4 GBq versus the 29.6 GBq recommended in the package insert (54).
Response Evaluation with PRRT
Evaluating response to PRRT can be challenging. Morphologic criteria such as RECIST 1.1 are the most frequently used, but anatomic changes often lag behind functional changes in pheochromocytoma and paraganglioma and may be better detected using 68Ga-DOTATATE, 18F-FDG, or other functional PET radiopharmaceuticals (55). Another issue is pseudoprogression, in which 9% of patients with RECIST-stable disease evidenced transient increases in tumor size by more than 10% at 6 wk after treatment (56). Three months appears to be the optimal time to determine treatment response. Changes in clinical status should guide the timing and interval of reevaluation.
Side Effects and Toxicity Profile
Although the toxicity profiles for 90Y- and 177Lu-PRRT have been characterized in patients with NETs, we feel that the toxicity profile in pheochromocytoma and paraganglioma is similar (57). Nonetheless, patients with pheochromocytoma or paraganglioma are at higher risk for certain side effects such as catecholamine release syndrome and tumor lysis syndrome (13,14).
A long-term follow-up study of patients receiving 90Y-DOTATOC versus 177Lu-DOTATATE with nephroprotection in NET found a slightly greater drop in renal function over time for 90Y-DOTATOC than for 177Lu-DOTATATE (58), although the overall incidence was low. In the pivotal NETTER-1 trial of 177Lu-DOTATATE in gastroenteropancreatic NET, renal failure of all grades occurred in 12% of patients, with 3% having grade 3 or 4 toxicity. In pheochromocytoma and paraganglioma reports, approximately 9% had renal toxicity higher than grade 3 (59).
Hematologic side effects are usually mild. The incidence of anemia in the NETTER-1 trial was 81% overall, with no grade 3–4 events. Thrombocytopenia and neutropenia of any grade occurred in 53% and 26% of patients, with grade 3–4 events in 1% and 3%, respectively. Grade 3 and 4 decreases in lymphocytes are frequent but do not require dose modifications (60). However, myelodysplastic syndrome or acute leukemia was reported in 3.4% of patients with nonpheochromocytoma or nonparaganglioma (61). In patients with pheochromocytoma or paraganglioma treated with PRRT, the incidence of myelodysplasia is similarly low, ranging from 2.5% to 8.3% (14,62,63).
Summary
The available retrospective data considering PRRT in metastatic or inoperable pheochromocytoma and paraganglioma show promise. Although complete responses as defined by RECIST have not been reported, a significant number of patients are able to achieve partial responses, and many have stable disease. Side effects are generally mild and well tolerated. An ongoing prospective phase II clinical trial (NCT03206060) of patients with sporadic or SDHx-related metastatic or inoperable pheochromocytoma or paraganglioma with clear evidence of progression receiving 7.4 GBq of 177Lu-DOTATATE every 8 wk (4 cycles) is ongoing. Preliminary evaluation after 2 cycles of treatment using functional imaging in 11 patients has shown promising results (64). Until final results are available, the National Comprehensive Cancer Network recommendations indicate that the use of 177Lu-DOTATATE with the activity and schedule stated in the package insert may be beneficial to many patients with metastatic or inoperable pheochromocytoma or paraganglioma.
If tumor uptake of 177Lu-DOTATATE and 131I-MIBG are equally good, the selection of radiopharmaceutical for RPT will rely on other considerations (65).
CONCLUSION
Currently, 131I-MIBG and PRRT are 2 RPT approaches that have shown efficacy in patients with metastatic or inoperable pheochromocytoma or paraganglioma, with acceptable toxicity profiles. Once the decision to use RPT is made, one must decide which of these 2 approaches to pursue. The first consideration is based on the degree of tumor localization of radiolabeled MIBG or DOTA-somatostatin analog. Given that disparate imaging results are not uncommon, this consideration will often inform the selection. However, if both tracers demonstrate good localization, other considerations, including cost, whether the treatment is inpatient or outpatient, and preexisting organ toxicities such as renal abnormalities or marrow toxicity, must be considered to pair each patient with the optimal radiopharmaceutical.
Footnotes
↵* Contributed equally to this work.
Learning Objectives: On successful completion of this activity, participants should be able to (1) describe the clinical features of pheochromocytoma and paraganglioma, including common symptoms and signs, management options, imaging findings, and the role of genetic and hormonal biomarkers; (2) identify and compare radiopharmaceutical agents available for treatment of metastatic or inoperable pheochromocytoma and paraganglioma; and (3) identify differences between low-specific-activity and high-specific-activity 131I-MIBG.
Financial Disclosure: This research was funded in part through the NIH/National Cancer Institute Cancer Center support grant P30 CA008748 and Eunice Kennedy Shriver National Institutes of Child Health and Human Development grant Z1AHD008735, NIH. Daniel Pryma receives research funding from Siemens and 511 Pharma and consulting fees from Siemens, 511 Pharma, and Bayer. The authors of this article have indicated no other relevant relationships that could be perceived as a real or apparent conflict of interest.
CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, SAM, and other credit types, participants can access this activity through the SNMMI website (http://www.snmmilearningcenter.org) through September 2024.
- © 2021 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication November 2, 2020.
- Accepted for publication February 16, 2021.