Abstract
Boronophenylalanine has been applied in clinical boron neutron capture therapy for the treatment of high-grade gliomas. The purpose of this study was to evaluate the pharmacokinetics of 4-borono-2-18F-fluoro-l-phenylalanine-fructose (18F-FBPA-Fr) in F98 glioma–bearing Fischer 344 rats by means of intravenous injection of 18F-FBPA-Fr both with and without blood–brain barrier disruption (BBB-D) induced by focused ultrasound (FUS). Methods: Dynamic PET imaging of 18F-FBPA-Fr was performed on the ninth day after tumor implantation. Blood samples were collected to obtain an arterial input function for tracer kinetic modeling. Ten animals were scanned for approximately 3 h to estimate the uptake of 18F radioactivity with respect to time for the pharmacokinetic analysis. Rate constants were calculated by use of a 3-compartment model. Results: The accumulation of 18F-FBPA-Fr in brain tumors and the tumor-to-contralateral brain ratio were significantly elevated after intravenous injection of 18F-FBPA-Fr with BBB-D. 18F-FBPA-Fr administration after sonication showed that the tumor-to-contralateral brain ratio for the sonicated tumors (3.5) was approximately 1.75-fold higher than that for the control tumors (2.0). Furthermore, the K1/k2 pharmacokinetic ratio after intravenous injection of 18F-FBPA-Fr with BBB-D was significantly higher than that after intravenous injection without BBB-D. Conclusion: This study demonstrated that radioactivity in tumors and the tumor-to-normal brain ratio after intravenous injection of 18F-FBPA-Fr with sonication were significantly higher than those in tumors without sonication. The K1/k2 ratio may be useful for indicating the degree of BBB-D induced by FUS. Further studies are needed to determine whether FUS may be useful for enhancing the delivery of boronophenylalanine in patients with high-grade gliomas.
Boron neutron capture therapy (BNCT) is a binary cancer treatment system that requires the selective delivery of a boron-containing drug to the tumor and then irradiation with neutrons to yield high-linear-energy-transfer α particles and recoiling 7Li nuclei (1–5). Successful application of BNCT requires the selective delivery of 10B to the tumor, low levels in the surrounding tissue, and the delivery of sufficient thermal neutron fluence to the tumor site (6,7). Considerable efforts have been dedicated to the development of boron-delivering agents that could ensure a high tumor-to-normal tissue ratio of uptake of boron in tumors for BNCT (8). With the development of new techniques for the chemical synthesis of an effective agent, several new potential boron-delivering agents have emerged. However, drug delivery will have to be optimized because it is unlikely that any single agent will be capable of targeting most of the tumor cells.
Despite the fact that the blood–tumor barrier is more permeable than the blood–brain barrier (BBB), the efficacy of systemic chemotherapy in patients with brain tumors is poor because the selective permeability of the blood–tumor barrier still restricts the accumulation of drugs delivered to the tumors (9). Promising clinical results for the treatment of high-grade gliomas have been reported by several clinical groups in Japan (10–13), Sweden (10,14), and Finland (15). Barth et al. demonstrated that boron uptake in brain tumors and animal survival could be improved by BBB disruption (BBB-D) induced with a hyperosmotic solution of mannitol (16,17). It has been shown that the amount of boron in brain tumors and the tumor-to-normal brain ratio after BBB-D induced by mannitol injection are significantly higher than those for tumors without BBB-D (18). Although this approach induces BBB-D throughout the entire area of the brain supplied by the injected artery (19,20), the boron compound is rapidly cleared from the normal brain and persists at a higher level in the tumor.
Our previous studies showed that focused ultrasound (FUS) could noninvasively enhance the permeability of BBB in the local region (21–23). The degree of FUS-induced BBB-D is affected by several ultrasound parameters, including acoustic power, frequency, burst length, the duty cycle of the transducer, and the concentration and size of the ultrasound contrast agent (24–26). The use of therapeutic agents followed by sonication represents a feasible approach for enhanced local drug delivery and improved treatment efficacy in brain tumors (27,28). Moreover, it has been demonstrated that boronophenylalanine (BPA) injection in combination with FUS exposure increases the accumulation of boron in brain tumors (29). An evaluation of micro-SPECT/CT imaging showed that FUS not only significantly increased the permeability of the BBB at the sonicated site but also significantly elevated the tumor-to-normal brain ratio for a drug in the focal region (30,31). Nuclear imaging may be useful for offering a good assessment of the extent of BBB-D and identifying the optimum therapeutic window for radiotherapy or chemotherapy of brain tumors with BBB-D induced by sonication.
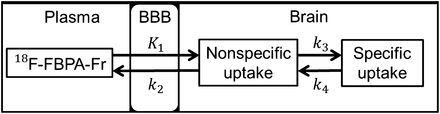
In this study, we evaluated the pharmacokinetics of 4-borono-2-18F-fluoro-l-phenylalanine-fructose (18F-FBPA-Fr) after intravenous injection, both with and without BBB-D induced by FUS, in F98 glioma–bearing rats. The pharmacokinetics of 18F-FBPA-Fr after sonication were monitored with noninvasive PET to demonstrate the optimal treatment protocol for thermal neutron irradiation. Furthermore, the rate constants K1, k2, k3, and k4 were derived from dynamic small-animal PET images (Fig. 1).
Pharmacokinetics of 18F-FBPA-Fr analyzed by 3-compartment model for K1 (mL/g/min), k2 (min−1), k3 (min−1), and k4 (min−1). K1 and k2 represent forward transport and reverse transport of 18F-FBPA-Fr across BBB, respectively. k3 and k4 are anabolic and reverse-process rate constants, respectively.
MATERIALS AND METHODS
Glioma Tumor Model and 18F-FBPA-Fr Preparation
Male Fischer 344 rats (11–13 wk; approximately 250–280 g) were anesthetized with an intraperitoneal administration of pentobarbital at a dose of 40 mg/kg of body weight. Next, 105 F98 rat glioma cells in 10 μL of Hanks balanced salt solution without Mg2+ and Ca2+ were injected into the brain. The glioma cells were stereotactically injected into a single location in the right hemisphere (5.0 mm posterior and 3.0 mm lateral to the bregma) of each rat at a depth of 5.0 mm from the brain surface. Next, the holes in the skull were sealed with bone wax, and the wound was flushed with iodinated alcohol. All procedures were performed according to the guidelines of and were approved by the Animal Care and Use Committee of the National Yang-Ming University. 18F-FBPA-Fr was prepared by the method described in a previous report (32). The radiochemical purity of 18F-FBPA-Fr was greater than 97%.
Focused Ultrasound System and Exposure
Pulsed FUS exposures were generated with a 1.0-MHz, single-element focused transducer (A392S; Panametrics). The entire FUS system setup was described in our previous study (33). The half maximum of the pressure amplitude of the focal zone had a diameter and a length of 3 mm and 26 mm, respectively. The ultrasound contrast agent (SonoVue; Bracco International) was injected into the tail vein of the rats approximately 15 s before sonication. The sonication was precisely targeted with a stereotactic apparatus (Stoelting) that used the bregma of the skull as an anatomic landmark. Sonication was applied to the tumor region of 5 glioma-bearing rats on day 9 after tumor cell implantation. The focal zone of the ultrasound beam was delivered to a location in the right brain hemisphere centered at the tumor injection site. The sonication parameters were as follows: an acoustic power of 2.86 W (corresponding to a peak negative pressure of 0.7 MPa) with an ultrasound contrast agent injection of 300 μL/kg, a pulse repetition frequency of 1 Hz, a duty cycle of 5%, and a sonication time of 60 s.
Dynamic PET Imaging
Dynamic small-animal PET images of the F98 glioma–bearing rats were obtained with a FLEX Triumph preclinical imaging system (Gamma Medica-Ideas, Inc.). The sensitivity and spatial resolution of the FLEX Triumph PET/SPECT/CT scanner were 1.1% and 0.91 mm, respectively. Animals were anesthetized by inhalation of 2% isoflurane in oxygen at 2 L/min in the prone position. Small-animal PET/CT was performed on day 9 after tumor cell implantation. For evaluation of the effect of BBB-D induced by FUS, each rat received a bolus injection into the tail vein of 18F-FBPA-Fr at a dose of 22.2 MBq for both brain tumors with BBB-D and brain tumors without BBB-D. Dynamic data acquisition was obtained with several frames at short intervals, followed by five 30-min frames up to 3 h after injection. The images were viewed and quantified with AMIDE (A Medical Imaging Data Examiner) software (free software provided by SourceForge) (34). Spheric regions of interest (radius, 2.5 mm) under the skull defect were manually pinpointed at the sonicated site and in the same region of the contralateral brain. The mean radioactivity within the region subjected to BBB-D at various time intervals was determined and compared with the results obtained for the equivalent region of the contralateral brain. Time–activity curves were plotted for both the tumor and the contralateral (normal) brain.
Pharmacokinetic Analysis
Arterial blood samples were obtained during dynamic small-animal PET scanning; a total of 14 samples (0.1 mL each) were collected over a period of 3 h. The blood was spun in a microcentrifuge, and red cells and plasma were separated for inherent circulation plasma substrate measurements for each animal. The radioactivity of plasma samples was assayed with a γ scintillation counter and was expressed in kBq/mL of plasma sample. The pharmacokinetic parameters of the tissue could be estimated from the dynamically acquired small-animal PET images. with the data for plasma sample radioactivity as the arterial input function. The pharmacokinetics of 18F-FBPA-Fr were analyzed by use of a modified 3-compartment model for K1 (mL/g/min), k2 (min−1), k3 (min−1), and k4 (min−1) (Fig. 1) (35). The rate constants (K1, k2, k3, and k4) were calculated by nonlinear regression with PMOD software (version 3.0; PMOD Technologies).
Histology
Two glioma-bearing rats from the FUS exposure group and 2 tumor-bearing control rats (no FUS exposure) were sacrificed after dynamic PET scanning for histologic examination. The rats were perfused with saline and 10% neutral buffered formalin. The brains were removed, embedded in paraffin, and then serially sectioned into 6-μm-thick slices. The slices were stained with hematoxylin and eosin to visualize the general cellular structure. Staining by terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) (DeadEnd Colorimetric TUNEL system; G7130; Promega) was performed to detect DNA fragmentation and apoptotic bodies within the cells. Photomicrographs of brain sections stained with hematoxylin and eosin and by TUNEL were obtained by use of a Mirax Scan digital microscope slide scanner (3D Histech) with a Plan-Apochromat 20×/0.8 objective (Carl Zeiss). The serial histology images were annotated with Pannoramic Viewer software (3D Histech).
Statistical Analysis
All data are shown as mean ± SEM. Statistical analysis was performed with an unpaired Student t test. The level of statistical significance was set at a P value of less than or equal to 0.05.
RESULTS
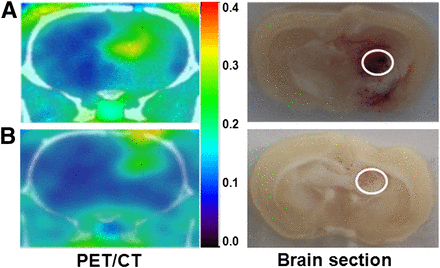
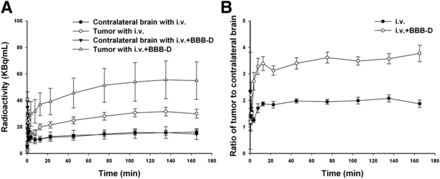
In the small-animal PET/CT images of glioma-bearing rats, high contrast was seen in brain tumors both with and without sonication but especially in the sonicated tumors (Fig. 2). Moreover, the corresponding brain sections were observed for tumor identification after small-animal PET/CT scanning (Fig. 2). Hemorrhage in the tumor region was due to BBB-D after FUS exposure. The time–activity curves derived from dynamic small-animal PET/CT images for 18F-FBPA-Fr in the tumor and the contralateral (normal) brain are shown in Figure 3A. No significant differences in 18F-FBPA-Fr uptake were found in the tumor and the contralateral brain despite the BBB-D for the tumor. Compared with the contralateral brain, the tumor showed a significant accumulation of radioactivity. Furthermore, the uptake of 18F-FBPA-Fr in the tumor was significantly increased by BBB-D after FUS. Both of the derived tumor-to-contralateral brain ratios reached a plateau at 15 min after 18F-FBPA-Fr administration (Fig. 3B). The mean tumor-to-contralateral brain ratios from 15 min to 165 min after intravenous injection of 18F-FBPA-Fr both with and without BBB-D were approximately 3.5 and 2.0, respectively.
Small-animal PET/CT images of F98 glioma–bearing Fischer 344 rats and corresponding brain sections of tumors both with (A) and without (B) sonication by FUS. Regions targeted by FUS are circled in tumors in right hemisphere.
Pharmacokinetics of 18F-FBPA-Fr in glioma-bearing rats, calculated from small-animal PET. (A) Time–activity curves for 18F-FBPA-Fr in tumors and contralateral brains of glioma-bearing rats. Data were obtained from dynamic small-animal PET images after intravenous (i.v.) injection of 18F-FBPA-Fr both with and without BBB-D. (B) Tumor-to-contralateral brain ratio for 18F-FBPA-Fr, derived from data in A. Each point represents mean ± SEM for 5 rats.
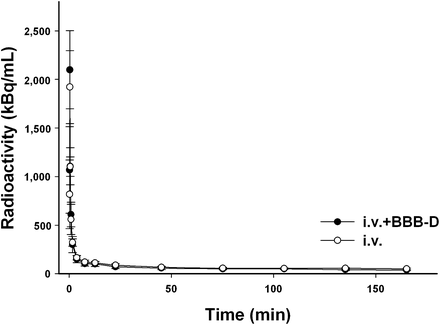
No significant differences in the time–activity curves for 18F-FBPA-Fr in plasma after intravenous injection were found both with and without BBB-D. These data were used as the arterial input function for calculating the pharmacokinetic parameters by use of tracer kinetic modeling (Fig. 4). Each point on the curve represented plasma sampling at a specific point during the time frame. The rate constants K1, k2, k3, and k4 derived from the arterial input function for the intravenous injection of 18F-FBPA-Fr with BBB-D are shown in Table 1.
Rate Constants for Brain Tumors After Intravenous Injection of 18F-FBPA-Fr Both With and Without FUS-Induced BBB-D
Time–activity curves for 18F-FBPA-Fr in plasma of F98 glioma–bearing rats after intravenous (i.v.) injection of 18F-FBPA-Fr both with and without BBB-D. Each point represents mean ± SEM for 5 rats.
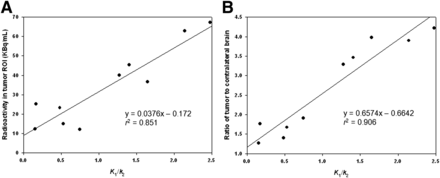
Next, the K1/k2 ratio was obtained from a pharmacokinetic analysis of blood from the same rat as that used for the data shown in Table 1. Regression analysis (Fig. 5A) revealed a linear relationship between the radioactivity in the tumor and K1/k2, quantified as tumor radioactivity (KBq/mL) = 0.0376 × K1/k2 − 0.172 (R2 = 0.851). Figure 5B shows the linear regression of K1/k2 with the tumor-to-contralateral brain ratio, quantified as tumor-to-contralateral brain ratio = 0.6574 × K1/k2 − 0.6642 (R2 = 0.906).
Correlations of K1/k2 ratio with radioactivity in tumor region of interest (ROI) and tumor-to-contralateral brain ratio. (A) Correlation between K1/k2 ratio and radioactivity in tumor ROI. (B) Correlation between K1/k2 ratio and tumor-to-contralateral brain ratio. Each point represents 1 rat. Mean radioactivity in tumor ROI and tumor-to-contralateral brain ratio were derived from small-animal PET images after intravenous injection of 18F-FBPA-Fr both with and without BBB-D.
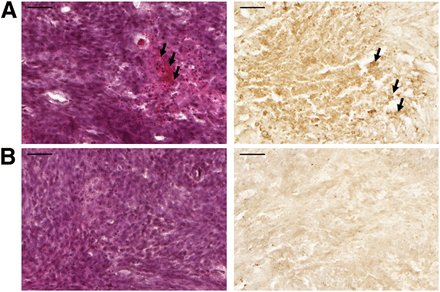
Figure 6 shows mild scattered extravasation of red blood cells and TUNEL-positive apoptotic cells in the tumor tissues treated with 18F-FBPA-Fr and sonication relative to the tumor tissues treated with 18F-FBPA-Fr alone.
Histologic observations. Nine days after tumor implantation, glioma-bearing rats given 18F-FBPA-Fr both with (A) and without (B) sonication were sacrificed for staining with hematoxylin and eosin (left) and by TUNEL (right) (scale bar, 50 μm). Arrows indicate hemorrhages and apoptotic cells.
DISCUSSION
The success of BNCT will depend mainly on the differential uptake of boron in tumors and normal tissues. When BPA is complexed with fructose, its accumulation in tumors has been proven to increase because of enhanced solubility (36). Previous studies reported that the pharmacokinetics of 18F-FBPA-Fr were similar to those of BPA (18,37). 18F-FBPA-Fr showed specific tumor uptake in F98 glioma–bearing rats and could be used as a probe for BPA complexed with fructose in clinical BNCT. In the present study, we used noninvasive small-animal PET/CT imaging to investigate the pharmacokinetics of 18F-FBPA-Fr after intravenous administration both with and without BBB-D induced by FUS.
To allow more 18F-FBPA-Fr to diffuse into the brain, hyperosmotic BBB-D was applied in preclinical BNCT. Compared with BNCT alone, BNCT with BBB-D showed significantly increased treatment efficacy and animal survival (38,39). Detailed pharmacokinetics after intravenous and intracarotid injections of 18F-FBPA-Fr and BPA, both with and without osmotic BBB-D, were reported in our previous study (18). The amount of boron in the tumor and the tumor-to-normal brain ratio after intracarotid injection of BPA with osmotic BBB-D were significantly higher than those produced by other delivery routes without BBB-D. For BNCT to be successful, it is essential not only to maximize tumor boron uptake but also to minimize boron concentrations in normal brain tissue. Neurotoxicity attributable to BBB-D combined with BPA or sodium sulfhydryl borane never was observed. However, BBB-D more effectively targeted infiltrating tumor cells (40). Therefore, targeted BBB-D induced by FUS could provide a powerful approach for local enhancement of boron uptake in the tumor region with minimal damage in the surrounding normal brain tissue.
The quantitative PET data indicated that the tumor-to-contralateral brain ratio in a glioma-bearing rat was 1.75-fold higher after intravenous injection of 18F-FBPA-Fr with BBB-D than after intravenous injection without BBB-D (Fig. 3B). Intravenous injection of BPA with FUS-induced BBB-D may be a better delivery method because this route is safer than intracarotid injection with hyperosmotic BBB-D used in previous studies. However, the bioeffects of ultrasound on brain tissue are still unknown, and further investigations of FUS are needed.
The modified 3-compartment model shown in Figure 1 indicated that an increase in BBB permeability was promoted by K1 and suppressed by k2. After the intravenous injection of 18F-FBPA-Fr with BBB-D, a mild increase in K1 and a significant decrease in k2 were seen (Table 1). These data indicated that 18F-FBPA-Fr was transported from the brain into the blood more readily after FUS-induced BBB-D. The K1/k2 ratio was significantly higher (∼4.37-fold) after intravenous injection of 18F-FBPA-Fr with BBB-D than after intravenous injection without BBB-D. As described earlier, a decrease in k2 was the principal factor affecting the K1/k2 ratio of 18F-FBPA-Fr after FUS-induced BBB-D. High interstitial fluid pressure is known to reduce the driving force for extravasation in tumors despite large gaps in the endothelium and greatly confining the transport of drugs (41). The effect of FUS on the K1/k2 ratio was consistent with the hypothesis that the enhanced extravasation of 18F-FBPA-Fr resulted partly from the decrease in high interstitial fluid pressure after FUS exposure.
An important requirement for BNCT is the accumulation of boron compounds in the tumor. The tumor-to-normal tissue ratio is another key requirement for determining the optimal neutron irradiation time for BNCT to minimize irradiation of normal tissues. Analysis of the K1/k2 ratio with boron tumor uptake or the tumor-to-contralateral brain ratio after FUS-induced BBB-D revealed that the values exhibited a good correlation (Fig. 5). Furthermore, the results revealed that the intravenous injection of 18F-FBPA-Fr with BBB-D would result in a higher level of tumor uptake of BPA and a higher tumor-to-contralateral brain ratio. Thus, the K1/k2 ratio may offer an indication of the extent of FUS-induced BBB-D and provide excellent feedback information to the operator. In ongoing studies, we plan to attempt to enhance the efficacy of BNCT by FUS exposure in animal brain tumor models.
CONCLUSION
Investigation of the pharmacokinetics of 18F-FBPA-Fr with small-animal PET/CT imaging revealed that FUS not only significantly increased the accumulation of boron in the sonicated tumor but also significantly elevated the tumor-to-normal brain ratio in the focal region. The K1/k2 ratio could be used to evaluate boron uptake in the tumor and the tumor-to-normal brain ratio and could provide an indication of the extent of FUS-induced BBB-D. This noninvasive nuclear imaging method may be a promising quantitative approach for optimizing the therapeutic window in future applications of BNCT.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This study was supported by grants from the National Science Council of Taiwan (NSC 101-2320-B-010-036-MY3, NSC 102-2221-E-010-005-MY3, NSC 100-2321-B-010-010, and NSC 99-2321-B-010-017), the Cheng Hsin General Hospital Foundation (102F218C11 and 101F195CY18), the Taipei Veterans General Hospital (VGH99A-026 and VGH100A-045), the Biophotonics and Molecular Imaging Research Center, and the Ministry of Education Aim for the Top University Plan. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Feb. 13, 2014.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication May 2, 2013.
- Accepted for publication November 11, 2013.