Abstract
Targeting the prostate-specific membrane antigen (PSMA) with 68Ga-labeled and 18F-labeled PET agents has become increasingly important in recent years. Imaging of biochemically recurrent prostate cancer has been established as a widely accepted clinical indication for PSMA ligand PET/CT in many parts of the world because of the results of multiple, primarily retrospective, studies that indicate superior detection efficacy compared with standard-of-care imaging. For high-risk primary prostate cancer, evidence is growing that this modality significantly aids in the detection of otherwise occult nodal and bone metastases. For both clinical indications in recurrent as well as in primary prostate cancer, preliminary data demonstrate a substantial impact on clinical management. Emerging data imply that intraprostatic tumor localization, therapy stratification, and treatment monitoring of advanced disease in specific clinical situations might become future indications. Current criteria for image reporting of PSMA ligand PET are evolving given the expanding body of literature on physiologic and pathologic uptake patterns and pitfalls. This article intends to give an educational overview on the current status of PSMA ligand PET imaging, including imaging procedure and interpretation, clinical indications, diagnostic potential, and impact on treatment planning.
Prostate cancer (PC) is the most common cancer in men and the third most frequent cause of cancer-related death in men worldwide (1). After primary treatment, biochemical recurrence (BCR) occurs in approximately 30%–40% of patients. After potential salvage treatment options, patients are usually treated with androgen-deprivation therapy (ADT). Typically, after 2–8 y of ADT, prostate-specific antigen (PSA) begins to rise again, indicating metastatic castration-resistant PC, the lethal form of the disease.
In the primary setting, detection of extraprostatic spread is crucial for further treatment planning and determination of prognosis. However, the cross-sectional imaging and bone scintigraphy recommended in many guidelines have shown limitations in detecting sites of nodal or bone involvement in preoperative patients (2–4). Further, in patients with high suspicion of PC, multiparametric MRI helps to rule out clinically significant disease and to guide targeted biopsy (5), although multiparametric MRI can miss aggressive PC lesions (6). In BCR, accurate restaging is crucial because local versus systemic disease substantially influences further treatment management. Accurate diagnosis of the site and extent of disease can be used in tailoring potential salvage treatments; however, standard-of-care imaging also has sensitivity and specificity limitations in this regard.
In contrast, the use of PET/CT, combining functional and morphologic information, for PC imaging has been increasing within the last decade. 18F-FDG is the most widely used radiotracer in oncologic PET/CT imaging; however, only a minority of PC (i.e., only aggressive, poorly differentiated, or undifferentiated PC) shows a high glycolytic rate, limiting the use of 18F-FDG PET (7,8). In Europe, radiolabeled choline derivatives (18F-fluorocholine or 11C-choline) were among the most commonly used PET tracers for PC imaging. They were most frequently used for restaging of PC and primary staging in selected cases (e.g., high-risk PC). Detection and localization of primary PC are limited by nonspecific uptake in benign intraprostatic pathologies (9). Recent metaanalyses reported a high specificity of 95% but a poor sensitivity of 49% in primary nodal staging (10). Detection rates are positively associated with PSA level but are low (<50%) in patients with early BCR (i.e., PSA < 2 ng/mL) (11). Other PET radiopharmaceuticals have been investigated (e.g., 11C-acetate) or even Food and Drug Administration–approved (e.g., 18F-fluciclovine), in part demonstrating superiority over choline derivatives (12–15).
Given the limitations of the most widely investigated PET tracers, targeting the prostate-specific membrane antigen (PSMA) with molecular imaging agents has recently been increasingly investigated. PSMA is a transmembrane protein that is highly overexpressed (100- to 1,000-fold) on almost all PC tumors (16–19). Only 5%–10% of primary PC lesions have been shown to be PSMA-negative (20,21). PSMA expression levels increase with higher tumor stage and grade (16,18,22). Presently, the only Food and Drug Administration–approved PSMA agent is a radiolabeled anti-PSMA antibody (ProstaScint, capromab pendetide; EUSA Pharma); however, this targets an intracellular epitope of PSMA (7E11) (19) that cannot be accessed in viable tumor cells, limiting diagnostic performance (23).
In contrast, small-molecule PSMA ligands bind to the active site in the extracellular domain of PSMA and are internalized and endosomally recycled, leading to enhanced tumor uptake and retention and high image quality (24–27). The most widely used 68Ga-labeled PSMA ligands for PET imaging are 68Ga-PSMA-11 (68Ga-PSMA-HBED-CC) and the theranostic agents 68Ga-PSMA-617 and 68Ga-PSMA-I&T (28,29). 18F-labeled agents include 18F-DCFBC (30,31), 18F-DCFPyL (32), and 18F-PSMA 1007 (33). They exploit the average lower positron range (reducing blurring effects), longer half-life, and potential for centralized production and distribution of 18F compared with 68Ga. A tabular overview of the most common PSMA ligands in clinical use was recently published (34).
This article intends to give an educational overview on the current status of PSMA ligand PET, including imaging procedure and interpretation, clinical indications, diagnostic potential, and impact on treatment planning.
MAIN CLINICAL INDICATIONS OF PSMA LIGAND PET/CT AND CURRENT EVIDENCE IN THE LITERATURE
Biochemical Recurrence
Approximately 30%–40% of patients will fail primary treatment, with a rising PSA level indicating recurrent or metastatic disease. Depending on the localization and extent of disease and prior treatment, different salvage options are available. Salvage surgery or salvage radiotherapy is used for local and nodal recurrence; stereotactic radiotherapy, for oligometastatic disease or systemic treatment in disseminated disease. Therefore, accurate restaging is crucial in recurrent PC patients. Currently, imaging of BCR is the most clinically accepted and validated indication for PSMA ligand PET/CT. Although a prospective head-to-head comparison of 68Ga-PSMA ligands and choline derivatives is missing, several, mainly retrospective, studies investigating BCR patients showed a higher diagnostic efficacy for PSMA ligand than for choline derivatives (35–37). SUVmax and tumor-to-background ratios were superior for 68Ga-PSMA-11 compared with 18F-fluorocholine (35), and 68Ga-PSMA-11 showed a higher detection rate than 11C-choline for lymph nodes as well as bone metastases (37). Positive findings exclusively detected by 18F-fluorocholine PET/CT were rare (36). Three large retrospective studies (including 319, 248, and 1,007 patients, respectively) reported detection rates for 68Ga-PSMA-11 PET/CT in BCR of 88%, almost 90%, and 79.5%, respectively (38–40). In patients after curative treatment with a very low PSA level of less than 0.5 ng/mL the reported detection rate of PSMA ligand PET/CT ranged from 50% to 58% in different studies (36,38–40). A recent study including only patients after prior radiotherapy (median PSA of 5.8 ng/mL) presented detection rates of 33.3% for a PSA of less than 0.5 ng/mL, 71.4% for a PSA of 0.5 to less than 1 ng/mL, and 93.3% for a PSA of 1 to less than 2 ng/mL. Local recurrence after radiotherapy was reported in 71% of the cohort, and 40% had suspected lymph node metastasis (41). A first meta–regression analysis in a systematic review including 10 studies was recently published. It resulted in a predicted PSMA ligand PET/CT positivity rate of 42%, 58%, 76%, and 95% for PSA values of 0–0.2, 0.2–1, 1–2, and more than 2 ng/mL, respectively (42). However, the results of this analysis need to be interpreted with caution as different 68Ga-PSMA–based PET tracers were pooled and no systematic histologic verification was available. Figures 1 and 2 show examples of nodal and local recurrence detected by 68Ga-PSMA ligand PET/CT.
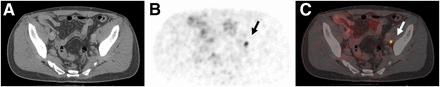
A 63-y-old patient with biochemical recurrence (PSA of 0.21 ng/mL) after radical prostatectomy (initially pT2c N0 M0 L1/V1 R1 G1, Gleason score of 7), local radiation treatment, and antiandrogen therapy. 68Ga-PSMA ligand PET/CT exhibits solitary left iliac radiotracer-positive lymph node (arrow). Shown are transaxial CT (A), PET (B), and fused PET/CT (C) images. Patient was referred for salvage lymph node dissection.
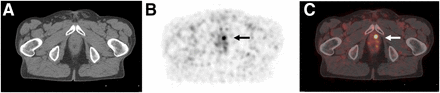
A 78-y-old patient with biochemical recurrence (PSA of 0.54 ng/mL) after radical prostatectomy (initially pT3b N0 M0 R0 G2). 68Ga-PSMA ligand PET/CT reveals focal uptake in left paramedian prostatic fossa, indicating local recurrence. Shown are transaxial CT (A), PET (B), and fused PET/CT (C) images. Patient was referred for salvage radiation treatment.
Studies evaluating 18F-labeled PSMA ligands suggest similar conclusions. In metastatic PC patients, the diagnostic performance of both 18F-DCFBC and 18F-DCFPyL PET/CT was superior to that of standard-of-care imaging for detecting suggestive lesions (32,43). In a head-to-head comparison in 14 patients with recurrent PC, staging with 18F-DCFPyL PET/CT was equivalent to that with 68Ga-PSMA-11 (44). A follow-up study from the same group using PSA-adjusted parallel biochemically recurrent PC patient cohorts (including a total of 191 patients) found that 18F-DCFPyL was noninferior to 68Ga-PSMA-11 (45) and suggested an improved sensitivity of the 18F-labeled radiotracer in the PSA range of 0.5–3.5 ng/mL (with the caveat that different injected doses and acquisition parameters were used for the 2 PSMA-targeted agents). Further in-depth clinical studies with standardized acquisition protocols and histologic validation are needed to establish the comparative performance of these 2 radiotracers.
Primary Staging
In high-risk PC patients, diagnosis of local extent and extraprostatic spread, that is, sites and extent of nodal and distant metastases, is crucial to further treatment planning (standard nodal dissection vs. extended dissection; change of primary radiotherapy field). Growing evidence underscores the role of PSMA ligand PET/CT imaging in primary PC, especially for N/M staging in a high-risk population. In detecting sites of nodal or bone involvement in preoperative patients, cross-sectional imaging has shown a limited pooled sensitivity and specificity of 42% and 82%, respectively, for CT and 39% and 82%, respectively, for MRI (3), as up to 80% of lymph node metastases in PC are harbored in normal-sized lymph nodes (2). Several studies showed a clear superiority of PSMA ligand PET/CT over standard-of-care imaging (CT, MRI, or bone scanning) (21,46–49). For example, in a retrospective analysis of 130 patients with primary intermediate- to high-risk PC using template-based pelvic histopathology as a reference, 68Ga-PSMA-11 PET performed significantly better than morphologic imaging for N staging both on a patient and a template basis (P = 0.002 and < 0.001, respectively). On template-based analysis, the sensitivity, specificity, and accuracy were 68.3%, 99.1%, and 95.2% for 68Ga-PSMA-11 PET and 27.3%, 97.1%, and 87.6% for morphologic imaging, respectively (21). Similar results on the diagnostic efficacy of PSMA ligand PET for the detection of nodal metastases were obtained in other studies (47,49). For bone metastases, Pyka et al. demonstrated that 68Ga-PSMA-11 PET significantly outperformed bone scanning because of both its high sensitivity and its high specificity on a patient and region basis (P = 0.006 and P < 0.0001, respectively). Because a histologic gold standard is not feasible in most cases for bone lesions, a best valuable comparator was defined on the basis of this study on a consensus review of all available current and follow-up images (including bone scanning/SPECT, PET, CT, MRI) and clinical data (48).
With regard to intraprostatic tumor localization by 68Ga-PSMA-11 PET/CT, imaging findings were correlated with histopathology using segment- or voxel-based approaches in several studies (50–52). These studies demonstrated relatively similar results, with a significantly higher 68Ga-PSMA-11 uptake in positive segments than in negative segments (SUVmax of 11.8 vs. 4.9 and 11.0 vs. 2.7, respectively, P < 0.001 each) (50,51). Results from combining 68Ga-PSMA-11 and multiparametric MRI on 53 preoperative intermediate-/high-risk patients indicated a potential for targeting biopsies. Hybrid 68Ga-PSMA-11 PET/MRI significantly outperformed multiparametric MRI and 68Ga-PSMA-11 PET in sensitivity and specificity for tumor localization on a sextant basis (respectively, 76% and 97% for hybrid 68Ga-PSMA-11 PET/MRI, 58% and 82% for multiparametric MRI, and 64% and 94% for 68Ga-PSMA PET) (53).
Using 18F-labeled compounds in a first cohort of 13 patients, the sensitivity of MRI in the detection of primary PC was superior to that of 18F-DCFBC PET/CT; however, 18F-DCFBC PET/CT demonstrated a higher specificity for clinically significant disease (31). The relatively low sensitivity of 18F-DCFBC in this context was likely at least partially attributable to its high blood-pool activity and low tumor-to-background ratios in relation to other small-molecule PSMA-targeted ligands, limitations potentially addressed by newer 18F-labeled agents such as 18F-DCFPyL and 18F-PSMA 1007. A first retrospective study using 18F-PSMA 1007 implied its high diagnostic potential by correctly detecting 18 of 19 histopathologically validated lymph node metastases in 8 patients with primary PC (33).
Advanced Disease
Typically, after 2–8 y of ADT the rise of PSA heralds the onset of metastatic castration-resistant PC, which is the lethal form of the disease and requires further systemic treatment (second ADT and taxane-based chemotherapy). The role of choline PET/CT in monitoring of systemic treatment in metastatic castration-resistant PC has been investigated in previously published studies and is still under debate (54,55). Sclerotic bone metastases are not regarded as “target lesions” using RECIST 1.1, and bone scintigraphy suffers from the well-known flare phenomenon. As preclinical data show that changes in PSMA expression can indicate the therapeutic success of taxane-based therapy (56), PSMA ligand PET/CT might overcome many of the limitations of standard-of-care imaging. However, ADT might represent a potential confounder due to temporal PSMA upregulation after initiation, followed by downregulation and finally gross overexpression in androgen-resistant tumors as has been found in preliminary studies (57–60).
Monitoring systemic treatment in certain clinical scenarios may become a future indication for PSMA ligand imaging; however, evidence is currently still sparse (61). PSMA ligand PET/CT has an evolving role in PSMA-targeting treatments (e.g., radioligand therapy), evaluating target expression and therefore potentially predicting response (62–64). A rare but potential limitation is absent or low PSMA expression (e.g., in visceral metastases) in advanced disease, which may be related to therapy-induced specific biologic subtypes (e.g., neuroendocrine differentiated PC) (65,66). Further information on the use of PSMA ligands for diagnosis has been published elsewhere (67,68).
IMPACT ON TREATMENT PLANNING
Treatment management of PC is highly associated with the site and extent of disease (local/nodal vs. systemic disease). Several studies have investigated the impact of PSMA ligand PET/CT on patient management and therapy. Most studies have focused on the value of PSMA ligand PET/CT in patients with BCR after curative treatment and have reported changes in therapeutic management depending on the specific clinical scenario and the extent of treatment modification (69–74). Most recently, an overall change in the therapeutic management of 75% of 131 patients after primary treatment was shown (69). Similar results were found in a smaller cohort of 45 patients, resulting in a change of treatment in 19 of 45 patients (42.2%), including extension of radiotherapy field or administration of dose escalation subsequent to local recurrence. In 2 of 19 patients, salvage radiotherapy was replaced by systemic treatment due to multiple metastatic lesions (70). In a well-defined patient cohort before salvage radiotherapy, a major management change in 20 of 70 patients (28.6%) with a PSA level of less than 1 ng/mL was demonstrated by van Leeuwen et al. (74).
In the setting of primary treatment, a small cohort of 15 patients underwent PSMA ligand PET/CT, and the imaging was found to influence clinical TNM stage in 53.3% of patients and radiotherapy plan in 33.3% (71). Combining the settings of radiotherapy planning in both primary and recurrent disease, 2 recent publications reported PSMA ligand PET/CT to have an impact on 50.8% and 53.7% of patients (72,73).
PSMA ligand PET/CT may also be used to guide salvage lymph node dissection, an emerging concept that may spare some patients ADT in early BCR. With the rise of PSMA ligand PET/CT, there is increasing interest based on both the high specificity and the improved sensitivity for detection of recurrent disease. Rauscher et al. have demonstrated high specificity (>95%) and superior sensitivity (78%) compared with standard-of-care imaging (27%) in patients who underwent salvage lymph node dissection (75). Preliminary results showed the feasibility of radioguided surgery exploiting preoperative labeling of lymph node metastases with a γ-emitting PSMA ligand (e.g., 111In-PSMA I&T), allowing detection and resection of even very small metastatic lesions (76,77). The recent introduction of 99mTc-PSMA I&S may facilitate dissemination of this promising technique (78).
PATIENT PREPARATION AND PSMA LIGAND PET/CT IMAGE ACQUISITION
Patient Preparation
Patients should be well hydrated before the study and during the uptake time (e.g., 500 mL of water orally during a 2-h period before acquisition). To reduce artifacts due to high tracer activity in the urinary system (potentially resulting in halo artifacts and false-positive findings), it is beneficial to coinject furosemide at the time of tracer injection and to have the patient empty the bladder immediately before image acquisition (79). Rectal filling with a negative contrast agent (100–150 mL) is optional to improve anatomic delineation of the rectum and differentiation of such structures as lymph nodes and seminal vesicles from adjacent structures.
Image Acquisition
68Ga-labeled PSMA ligands are applied intravenously using a recommended activity of 2 MBq per kilogram of body weight. 68Ga-PSMA-11 PET/CT is routinely conducted 1 h after injection according to its first described clinical set-up (27). However, the same article already demonstrated that late imaging conducted at 3 h after injection shows most PC lesions with higher contrast because of an ongoing decrease in background signal and increase in tracer uptake. Recently, one study demonstrated that the higher uptake and contrast of PC lesions in scans at 3 h after injection result in a higher number of lesions detected by 68Ga-PSMA-11 PET/CT and a higher number of patients with an overall positive PET result (80). In contrast, most recently, another large retrospective study showed no clear advantage of delayed imaging (81). However, delayed imaging might be considered in cases of equivocal findings or in the context of low PSA levels. Imaging with 18F-labeled agents has been described at 60 min after injection and 120 min after injection, with preliminary evidence indicating an improvement in lesion detection with later-time-point imaging (32,82).
Depending on previous imaging, either a low-dose or a diagnostic CT scan with or without intravenous contrast agent is performed. The PET acquisition should be performed in 3-dimensional mode with an acquisition time of 3–4 min per bed position. Technical details on correction of emission data, image reconstruction, and postprocessing for 68Ga-labeled PSMA ligands have been recently published (79).
PRACTICAL ISSUES
Image Display and Reading
Hybrid PET/CT image review is recommended on a dedicated postprocessing workstation allowing parallel visualization of PET, CT, and fused PET/CT images in the axial, coronal, and sagittal planes as well as maximum-intensity projections (3-dimensional cine mode). PET and CT should be linked at the same table position to help localize PET-positive findings. For PET interpretation, both uncorrected and attenuation-corrected images need to be assessed to identify artifacts (e.g., from contrast agents, metal implants, or patient motion). Further, dynamic variation of SUV threshold by changing display windowing is necessary to adjust such variables as the uptake of PSMA ligands in or adjacent to organs with high background uptake, such as the kidneys, ureter, or urinary bladder. Otherwise, findings such as local recurrence near the urinary bladder might be missed. Semiquantitative information on suspected lesions (SUVmean/max) can be derived on all slices of the attenuation-corrected PET study using a 3-dimensional volume of interest. Notably, there currently are no stringently defined SUV thresholds that reliably aid in differentiation between benign and malignant lesions. Diagnostic contrast-enhanced CT should be evaluated separately according to established radiologic criteria on a dedicated postprocessing workstation.
Physiologic PSMA Uptake and Variants
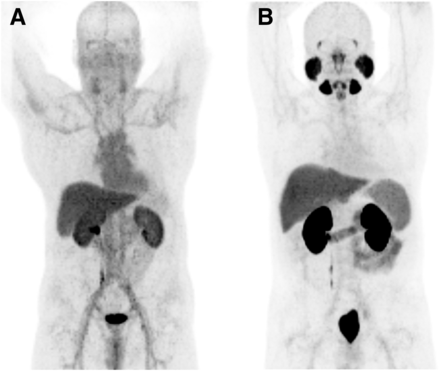
All low-molecular-weight PSMA ligands for PET imaging demonstrate typical physiologic PSMA ligand uptake in the lacrimal glands, parotid glands, submandibular glands, liver, spleen, small intestine, kidneys, and colon (Fig. 3). Notably, PSMA ligand uptake in the salivary gland is not definitively proven to be related to PSMA expression in the tissue. In addition, PSMA is synonymous with N-acetyl-l-aspartyl-l-glutamate peptidase I, which is an enzyme expressed in human brain tissue and has a role in regulating glutamate concentration.
Maximum-intensity-protection images (acquired with 18F-DCFBC [A] and 18F-DCFPyL [B]) displaying typical PSMA ligand biodistribution. Physiologic accumulation is seen in lacrimal and salivary glands, nasal mucosa, liver, spleen, bowel, kidneys, ureter on right side, and bladder.
All 68Ga- and 18F-labeled PSMA ligands are excreted via the kidneys, with subsequent high radiotracer uptake in the kidneys and the collected urine (16). Limited preliminary data indicate that 18F-PSMA 1007 might have reduced urinary clearance within the first 2 h after injection, potentially allowing for improved assessment of the prostate within this time window (33).
Pathologic PSMA Uptake Related to PC and Metastases
The excellent specificity of PSMA ligands, especially for lymph node metastases, was demonstrated in several studies (21,39,46,83–85). Therefore, any focal uptake of the PSMA ligand higher than the surrounding background uptake in morphologically visible lesions and not associated with physiologic uptake should be considered suggestive.
The pathologic uptake should be reported as low, moderate, or intense by comparison to the background uptake—for example, liver or spleen—as recently described (86). Besides local involvement (primary tumor vs. local recurrence), the typical metastatic pattern is primarily the regional pelvic lymph nodes. This is often followed by distant lymph nodes (above the aortic bifurcation) and bone metastases. In advanced disease, PC can even spread to the liver, lungs, or other visceral organs.
Limitations and Pitfalls in Clinical Interpretation
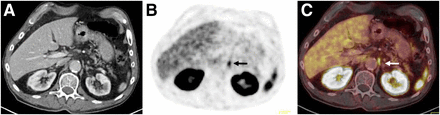
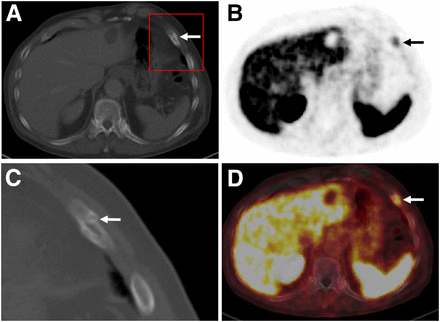
It is well known that the neovasculature of many solid tumors can also expresses PSMA (22). Accordingly, there is increasing evidence that PSMA ligand uptake is not exclusively specific for PC. A large number of case series and reports describe increased PET signal in benign lesions (e.g., neurogenic tissue, Paget disease, thyroid adenoma, granulomatous disease, and adrenal adenoma) as well as in malignant diseases (e.g., renal cell carcinoma, lung cancer, glioblastoma, hepatocellular carcinoma, and thyroid cancer) (87–95). Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org) summarizes the current evidence in the literature. Because many of these potential pitfalls can be solved in clinical context or by adding further imaging, increased PSMA ligand uptake in ganglia is the most common pitfall (Fig. 4). Their sites (especially sacral and celiac) are near the typical locations of lymph node metastases. Thus, knowledge of the CT characteristics (e.g., size, shape, and specific location) is crucial for reliable differentiation. In a recent investigation, at least one celiac ganglion with increased PSMA ligand uptake mimicking retroperitoneal lymph node disease was found in 89% of patients undergoing 68Ga-PSMA-11 PET/CT examinations (96). Another important limitation is the absence of PSMA overexpression in the primary tumor or its metastases in up to 10% of patients with primary PC or (as mentioned above) decreased PSMA expression in advanced disease (21). Side-by-side interpretation of the diagnostic CT scan as part of the PSMA ligand PET/CT examination is important. An example of a patient with a PSMA-positive rib fracture can be seen in Figure 5.
Transaxial CT (A), PET (B), and fused PET/CT (C) 68Ga-PSMA ligand scans demonstrating moderate, focal PSMA ligand uptake (arrows) in comma-shaped soft-tissue structure between left adrenal gland and aorta, indicating normal variant uptake in celiac ganglion.
68Ga-PSMA ligand PET/CT scan exhibiting moderate, focal PSMA ligand uptake in left rib on transaxial PET (B) and fused PET/CT (D) images. (A and C) Corresponding CT images confirm minimally displaced fracture of rib.
Current Regulatory Status for PSMA Ligands
Currently, PSMA ligands are not approved for clinical use in any country. In many European countries (especially Germany and Austria) the use of nonapproved agents for PET imaging is possible within certain limitations. For this use, the number of sites offering PSMA ligands is currently increasing because many institutions are evaluating PSMA ligands in prospective trials for either staging or restaging of PC. Most of the protocols for 68Ga-PSMA-11 are harmonized under a multicentric approach headed by the Clinical Trials Network of the Society of Nuclear Medicine and Molecular Imaging. This harmonization is intended to trigger a new drug application and potential Food and Drug Administration approval. In addition, 18F-DCFPyL is currently in multicenter phase II/III trials. Finally, an increasing number of clinical guidelines adopt the use of PSMA ligand PET, especially for BCR.
CONCLUSION
PSMA ligand PET/CT has become a clinically accepted technique for PC imaging worldwide and provides high diagnostic efficacy in recurrent PC as well as in staging of high-risk PC. Evidence is emerging that PSMA ligand PET/CT substantially influences treatment decisions by detection of sites of recurrence and nodal or distant metastases that are often occult on standard-of-care imaging. Intraprostatic tumor localization, therapy stratification, and treatment monitoring of advanced disease are potential future indications. Standardized criteria for image interpretation of PSMA ligand PET are evolving, facilitating its use in clinical practice. Several prospective trials are under way to support final market approval and reimbursement.
Footnotes
Published online Jul. 7, 2017.
Learning Objectives: On successful completion of this activity, participants should be able to (1) recognize the current status of PSMA ligand PET imaging (clinical indications, diagnostic value, impact on treatment planning), (2) apply PSMA PET/CT (patient preparation, image acquisition), and (3) interpret PSMA imaging.
Financial Disclosure: Martin G. Pomper is a coinventor on a U.S. Patent covering 18F-DCFPyL, and as such is entitled to a portion of any licensing fees and royalties generated by this technology. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. Martin G. Pomper and Steven P. Rowe have received research support from Progenics Pharmaceuticals, the licensee of 18F-DCFPyL. The authors of this article have indicated no other relevant relationships that could be perceived as a real or apparent conflict of interest.
CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA category 1 credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, SAM, and other credit types, participants can access this activity through the SNMMI website (http://www.snmmilearningcenter.org) through Xxxxxx 2020.
- © 2017 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.
- 14.
- 15.↵
- 16.↵
- 17.
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.
- 26.
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.
- 59.
- 60.↵
- 61.↵
- 62.↵
- 63.
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.
- 85.↵
- 86.↵
- 87.↵
- 88.
- 89.
- 90.
- 91.
- 92.
- 93.
- 94.
- 95.↵
- 96.↵
- Received for publication May 26, 2017.
- Accepted for publication July 6, 2017.