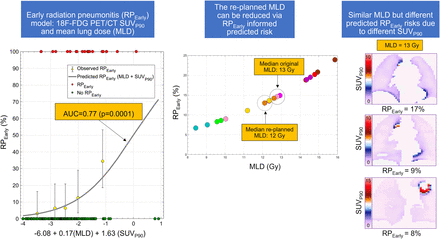
Visual Abstract
Abstract
Radiation pneumonitis (RP) that develops early (i.e., within 3 mo) (RPEarly) after completion of concurrent chemoradiation (cCRT) leads to treatment discontinuation and poorer survival for patients with stage III non–small cell lung cancer. Since no RPEarly risk model exists, we explored whether published RP models and pretreatment 18F-FDG PET/CT–derived features predict RPEarly. Methods: One hundred sixty patients with stage III non–small cell lung cancer treated with cCRT and consolidative immunotherapy were analyzed for RPEarly. Three published RP models that included the mean lung dose (MLD) and patient characteristics were examined. Pretreatment 18F-FDG PET/CT normal-lung SUV featured included the following: 10th percentile of SUV (SUVP10), 90th percentile of SUV (SUVP90), SUVmax, SUVmean, minimum SUV, and SD. Associations between models/features and RPEarly were assessed using area under the receiver-operating characteristic curve (AUC), P values, and the Hosmer–Lemeshow test (pHL). The cohort was randomly split, with similar RPEarly rates, into a 70%/30% derivation/internal validation subset. Results: Twenty (13%) patients developed RPEarly. Predictors for RPEarly were MLD alone (AUC, 0.72; P = 0.02; pHL, 0.87), SUVP10, SUVP90, and SUVmean (AUC, 0.70–0.74; P = 0.003–0.006; pHL, 0.67–0.70). The combined MLD and SUVP90 model generalized in the validation subset and was deemed the final RPEarly model (RPEarly risk = 1/[1+e(−x)]; x = −6.08 + [0.17 × MLD] + [1.63 × SUVP90]). The final model refitted in the 160 patients indicated improvement over the published MLD-alone model (AUC, 0.77 vs. 0.72; P = 0.0001 vs. 0.02; pHL, 0.65 vs. 0.87). Conclusion: Patients at risk for RPEarly can be detected with high certainty by combining the normal lung’s MLD and pretreatment 18F-FDG PET/CT SUVP90. This refined model can be used to identify patients at an elevated risk for premature immunotherapy discontinuation due to RPEarly and could allow for interventions to improve treatment outcomes.
The addition of immune checkpoint blockade (ICB) consolidation therapy after concurrent chemoradiation (cCRT) in patients with locally advanced non–small cell lung cancer (NSCLC) has significantly improved survival and represents the current standard of care (1–3). The ICB consolidation therapy is administered intravenously over 1 y and has led to a 3-fold increase in the median progression-free survival and a 10% absolute increase in the 5-y overall survival compared with the prior cCRT alone standard of care (4). However, ICB consolidation therapy has also been found to increase the incidence of symptomatic pneumonitis (1,5,6). Before the use of ICB consolidation, radiation pneumonitis (RP) would result in morbidity but was rarely associated with poor survival. However, the development of RP is increasingly important since it can now lead to the premature discontinuation of ICB consolidation therapy before the planned 1 y and is thereby associated with poorer survival (5,7). Furthermore, early RP (i.e., developing ≤3 mo after completion of cCRT; RPEarly) has recently been suggested as the most critical toxicity, as it can lead to a markedly insufficient duration of ICB therapy (7,8).
Models to predict the risk of RP have been developed to guide radiation therapy (RT) planning and inform patient counseling, but these models have been derived from patients treated with cCRT alone, and the models further underestimate the rate of RP in patients treated with cCRT and ICB (6). This is largely due to the higher rate of RP with the addition of ICB consolidation (6) but is also due to the limited ability of these models to accurately predict patients with RP even after cCRT alone (9). Given the increased use of ICB consolidation therapy and a growing number of strategies to use immunotherapy agents with cCRT (10), models that accurately estimate the risk of RP in the era of ICB therapy are warranted. In cohorts treated with cCRT alone, pretreatment 18F-FDG PET/CT imaging features are associated with increased RP risk (11). In noncancerous patients, such features have been indicative of pulmonary inflammation and hypothesized to be related to the density and activation of inflammatory immune cells in the lung (12,13).
There are currently no RP risk models specifically developed for RPEarly. In this study, we hypothesized that incorporating pretreatment 18F-FDG PET/CT features with RT dose would yield risk models able to successfully identify patients with an exacerbated risk of this new critical toxicity, RPEarly.
MATERIALS AND METHODS
Cohort
In total, 178 patients who had locally advanced NSCLC and were consecutively treated with cCRT and durvalumab between May 2017 and December 2021 at a multisite tertiary cancer center were reviewed, and the 160 patients with pretreatment 18F-FDG PET/CT scans available were included in this study. Patients avoided strenuous exercise for more than 24 h—and fasted for 6 h—before 18F-FDG injection. The required blood sugar level was less than 200 mg/dL. After 18F-FDG administration, the patients were instructed to rest quietly in the injection room. Before the study, the patients were asked to void the urinary bladder. 18F-FDG PET/CT was performed according to institutional guidelines, which are based on the joint European Association of Nuclear Medicine/Society of Nuclear Medicine and Molecular Imaging/European Society for Radiotherapy and Oncology practice recommendations for the use of 18F-FDG PET/CT for external-beam treatment planning in lung cancer (14). The PET/CT scans were acquired in 3 dimensions on a Discovery 690 or 710 PET/CT (GE Healthcare Inc.) (15). Patients were positioned on a flat RT tabletop. Whole-body PET acquisitions commenced about 60 min after administration of approximately 480 MBq of 18F-FDG, at 3 min/bed position. All PET emission data were corrected for attenuation, scatter, and random events and were iteratively reconstructed into a 128 × 128 × 47 matrix (voxel dimensions, 5.47 × 5.47 × 3.27 mm) using an ordered-subset expectation maximization algorithm (2 iterations, 16 subsets) incorporating time-of-flight and point-spread function modeling. A gaussian postprocessing filter of 6.4 mm in full width at half maximum was also applied. Respiratory motion correction was not performed.
Patients were prescribed 60 Gy in 2-Gy fractions concurrent with platinum doublet chemotherapy (6,16). Durvalumab consolidation was initiated at a median of 1.4 mo after cCRT completion (Table 1). All patients had standard follow-ups after treatment, with a history, physical examination, and chest CT scan being obtained every 3 mo for the first 2 y. RP of grade 2 or higher was defined as a patient’s having worsening pulmonary symptoms, including dyspnea or cough not attributable to other causes, within 12 mo from the completion of cCRT and having CT-based imaging changes within the irradiated field (5,17). Three RP endpoints were studied: 3 mo after completion of cCRT (RPEarly), more than 3 mo after completion of cCRT (RPLate) (Table 2), and the combination thereof (RPEarly+Late), which is the definition that has traditionally been used. Patients with clinical and imaging characteristics consistent with RP were retrospectively assessed for their clinical course of RP, and RP grading was based on the Common Terminology Criteria for Adverse Events, version 5.0. This retrospective study was completed under an institutional review board–approved protocol.
Patient Characteristics
Data for 38 Patients in Whom RP Developed
Modeling
Applying Published Risk Models to RPEarly
In our previous work (17), which focused on RP in the thorax after any type of ICB, 3 published RP models were identified and explored: QUANTEC mean lung dose (MLD) alone (18); MLD, age, obstructive lung disease, smoking status, and tumor location (19); and MLD, age, and obstructive lung disease (9). The MLD was extracted for the total normal lung (excluding the tumor) and converted to an equivalent dose in 2-Gy fractions, assuming 3 Gy for the fractionation sensitivity parameter α/β (EQD23) (18); the other model parameters were extracted from the medical records. Each published model’s coefficients were applied to the corresponding parameters (with no refitting). Model suitability was assessed both as calibration via the Hosmer–Lemeshow test (pHL; ideal value, 0.50) and as discrimination via the area under the receiver-operating characteristic curve (AUC; ideal value, 1.00) and P value (ideal value, 0). Each of the 3 published models was also studied for association with RPLate in addition to RPEarly+Late.
Integrating 18F-FDG PET/CT Features with Published Risk Models for RPEarly
To obtain 18F-FDG PET/CT features, the normal lung in the planning CT scan was propagated onto the low-dose CT scan using Plastimatch routines for b-spline–based deformable image registration within the computational environment for RT research (20). All propagated normal-lung contours were quality-controlled to limit the influence of registration uncertainties. The SUV was normalized with respect to the body mass. Since most second-order histogram lung 18F-FDG PET/CT features have previously been found to be nonreproducible across reconstruction algorithms (21–24), only first-order histogram features of the SUV were extracted: 10th percentile of SUV (SUVP10), 90th percentile of SUV (SUVP90), SUVmax, SUVmean, minimum SUV, and SD. These features adhered to the Image Biomarker Standardization Initiative (25) and were automatically extracted using the radiomics toolbox of the computational environment for RT research (26).
A TRIPOD type 2b model (27) was generated in which the 160-patient cohort was randomly split, but with similar RPEarly and RPLate rates, into a 112-patient subset (70%) used to build the model. The remaining 48-patient subset (30%) was used for internal validation of the built model. During model building, each feature was univariately associated with RPEarly using logistic regression with bootstrap resampling (repeated over 1,000 samples), and a candidate predictor was indicated by a P value of less than 0.05. The model parameters in the published models that were found to significantly predict RPEarly were refitted to the training data, and a new multivariate model was built with the published model parameters and the 18F-FDG PET/CT predictors. Again, the pHL, AUC, and P values were used to assess model suitability but now of the new combined 18F-FDG PET/CT and published model parameters.
RESULTS
Patient Treatment and RP Characteristics
In total, 38 (24%) of the 160 evaluated patients developed RP at a median of 3 mo (range, 1–9 mo) from cCRT completion (Table 1), initiated durvalumab significantly earlier than patients without RP (median, 41 vs. 45 d; P = 0.03; Table 1), and were initiated on steroid therapy. Twenty-four (63%) of the 38 patients with RP had resolution or near resolution of RP symptoms 3 mo from onset (Table 2), and of these, 6 (16%) patients were rechallenged with durvalumab, whereas the remaining patients permanently discontinued durvalumab.
Of the 38 patients who experienced RP, 20 (53%) patients did so as RPEarly and the remaining 18 (47%) as RPLate (Supplemental Table 1). Among patients with RPEarly, 10 (50%) remained on steroids 3 mo from symptom onset and the remaining 10 (50%) had resolution or near resolution of RP symptoms. Additionally, 4 (20%) patients with RPEarly were rechallenged with durvalumab (Table 2). The appearance of RP on the diagnostic CT scan along with the corresponding treatment planning CT scan to illustrate the RT field is given in Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org) for 1 representative patient who developed RPEarly and 1 representative patient who developed RPLate.
Assessment of Published RP Models in Predicting RPEarly
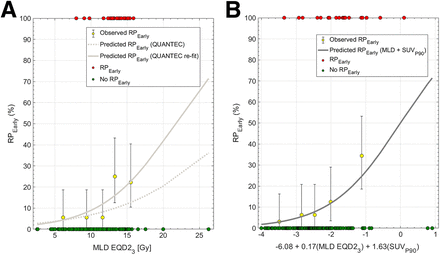
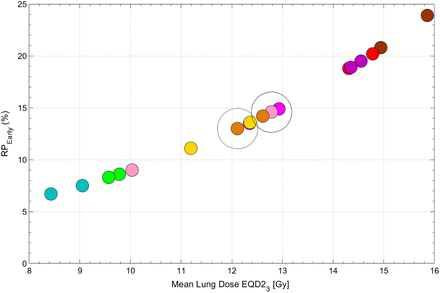
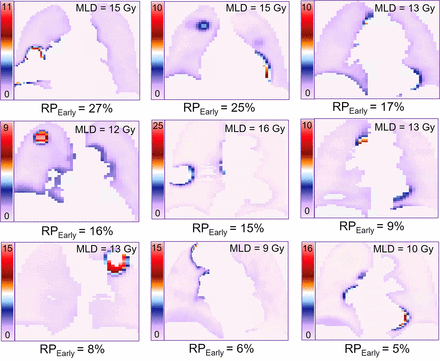
Among the 3 published models tested, only the QUANTEC MLD-alone model (18) significantly predicted RPEarly (AUC, 0.72; P = 0.02; pHL, 0.87; Fig. 1A); the other 2 models (9,19) did not (AUC, 0.62, 0.62; P = 0.10, 0.57). Refitting the MLD model improved calibration (pHL, 0.65 vs. 0.87). At the National Comprehensive Cancer Network, MLD of 20 Gy or less (MLD EQD23 ≤ 15 Gy), the predicted risk of RPEarly was 20%. To explore the feasibility of reducing MLD beyond this guideline, 9 of 20 patients with RPEarly were randomly selected for replanning of their RTs. Three treatment planners specializing in thoracic cancers each replanned 3 patients intending to maximally reduce MLD without compromising any other clinical treatment planning criteria. The MLD could be further reduced in all patients and to a median of 12 EQD23 Gy (range, 8–15 Gy in EQD23), compared with the original 13 EQD23 Gy (range, 9–16 EQD23 Gy), with individual differences ranging from 0.2 to 2.8 EQD23 Gy. This MLD reduction resulted in a 1%–6% decrease in predicted RPEarly (Fig. 2).
(A) Dose–response curve for QUANTEC’s MLD model (18) (dotted line) applied to RPEarly and refitted MLD model for RPEarly (solid line) in 160-patient cohort. Observed data are aggregated in quintiles (yellow; error bars: 95% binomial CIs), in addition to each observation stratified by RPEarly status. (B) Predicted dose–response curve combining MLD with SUVP90 in 160-patient cohort; observed data are aggregated as quintiles (yellow; error bars: 95% binomial CIs), in addition to each observation stratified by RPEarly status.
Predicted RPEarly based on refitted MLD model alone for random 9-patient subset with RPEarly in which replanning was performed. Each color represents each patient, and rightmost circle is MLD from original treatment plan; leftmost circle is MLD from replanning procedure. Population median MLD before and after replanning is denoted by larger circles (right: MLD from original plan; left: MLD after replanning).
Improved Ability to Correctly Identify RPEarly by Integrating 18F-FDG PET/CT with MLD
Among all patients, the median SUVmean and SUVmax were 0.65 (range, 0.03–1.9) and 10 (range, 0.09–27), respectively. With similar magnitudes of association, SUVmean, SUVP10, and SUVP90 significantly predicted RPEarly (AUC, 0.65–0.66; P = 0.003–0.006; pHL, 0.67–0.70). These 3 18F-FDG PET/CT features were all strongly intercorrelated (Spearman’s rho = 0.95). Therefore, bivariate models between MLD and each of these features were built. All bivariate models significantly predicted RPEarly in the derivation subset (AUC, 0.79–0.80; P = 0.0005–0.0008; pHL, 0.61–0.64), whereas only the model including MLD and SUVP90 generalized in the internal validation subset (AUC, 0.65, P = 0.03, pHL, 0.96). This model was, therefore, considered final. Thereafter and only to obtain model coefficients, the final model was refitted to the entire cohort (AUC, 0.77, P = 0.0001, pHL, 0.65; Fig. 2); the risk of RPEarly is given by the following logistic function equation: RPEarly risk = 1/[1+e(−x)]; x = −6.08 + [0.17 × MLD] + [1.63 × SUVP90]. In the riskiest model quintile, MLD and SUVP90 were 15 Gy and 1.51 (Fig. 1B: the rightmost bin), whereas in the least risky quintile, they were 6.7 Gy and 0.89 (Fig. 1B: the leftmost bin). The median SUVP90 among patients with RPEarly was 1.2 (range, 0.8–2.4), compared with 1.0 (range, 0.05–2.8) for patients without RPEarly. According to the bivariate MLD and SUVP90 model, the risk of RPEarly varies for MLDs of similar magnitude. For example, an MLD of 13 Gy is associated with a risk of RPEarly varying from 8% to 17% depending on a patient’s distribution of SUVP90 (Fig. 3).
Midcoronal slices of highest SUVP90 voxelwise distribution maps for 9-patient subset that developed RPEarly and were randomly selected for MLD-sparing replanning. MLD EQD23 is inserted for each patient in upper right corner.
No Risk Model Established for RPLate
None of the 3 published models (AUC, 0.44–0.52; P = 0.50–0.69; pHL, 0.85–0.99) or any of the 6 18F-FDG PET/CT features (AUC, 0.55–0.60; P = 0.14–0.58; pHL, 0.71–0.73) predicted RPLate. Similarly, no published model significantly predicted RPEarly+Late (AUC, 0.55–0.60; P = 0.07–0.98; pHL, 0.99–1.00), but all SUV parameterizations except SUVmax were significantly associated with RPEarly+Late (AUC, 0.64–0.67; P = 0.006–0.01; pHL, 0.64–0.66), which was likely driven by the stronger association between SUV features and RPEarly.
DISCUSSION
RPEarly in patients with locally advanced NSCLC can lead to the premature discontinuation of life-prolonging ICB consolidation therapy. To date, no risk prediction models have been tested for, or specifically tailored to, RPEarly. Herein, we present a novel model that combines the MLD with SUVP90 of the normal lung from pretreatment 18F-FDG PET/CT, which leads to an improved ability to identify the risk of RPEarly over using MLD alone (AUC, 0.72 vs. 0.77). Thus, these results suggest that patients at high risk of RPEarly could be identified by assessing the pretreatment SUVP90, which could thereby inform patient-specific treatments lowering the MLD to further mitigate RPEarly. Importantly, the MLD was found to predict RPEarly but not RPLate, suggesting that the risk of RPEarly, the most consequential RP in the setting of cCRT and ICB, can be directly mitigated and modified by limiting the MLD of the normal lung.
There is an increased risk of RP in patients who have locally advanced NSCLC treated with cCRT and ICB consolidation therapy compared with cCRT alone (5,28,29), with about 25% of patients treated with cCRT and ICB consolidation therapy expected to develop RP. However, only recently have data emerged that early discontinuation of ICB consolidation because of RP is associated with poorer survival and disease control (6–8). We have shown that RP models derived from patients treated with cCRT alone widely underestimate the rate of RP in patients treated with cCRT and ICB consolidation (5). Although there have been reports of specific RT dose–volume histogram metrics being associated with the risk of RP in patients treated with ICB consolidation (30,31), these studies have been limited by a small number of patients, leading to inconclusive, conflicting results. Without dose–volume histogram guidelines derived for patients receiving cCRT and ICB consolidation therapy, RT planning and delivery to limit the risk of RP, particularly RPEarly, are suboptimal. All 3 published RP models explored here focused on RP prior to the introduction of ICB consolidation (9,18,19). Interestingly, the QUANTEC MLD model (18) was found to be associated with RPEarly (AUC, 0.72; P = 0.04). The model (19) that in addition to MLD included age, chemotherapy, obstructive lung disease, smoking status, and tumor location did not predict RPEarly (AUC, 0.62; P = 0.57), and neither did the model (9) that included MLD, age, and obstructive lung disease (AUC, 0.62; P = 0.10). Taken together, the inability of published models that include patient characteristics to predict RPEarly in patients treated with cCRT and ICB consolidation therapy motivates the need to identify other relevant characteristics to improve the ability to accurately capture RPEarly. After thorough model building, the final model generated here that combined SUVP90 with MLD had an improved ability to predict RPEarly over using MLD alone.
The normal-lung SUV from 18F-FDG PET/CT has previously been found to be elevated among patients with chronic obstructive pulmonary disorder (32) and to be associated with inflammation in acute lung injury (13). The underlying mechanisms of a high SUV in the normal lung have been hypothesized to be attributed to increased density and baseline activation of inflammatory immune cells, as their activation is characterized by increased glucose utilization leading to an increased SUV. Since RP is a consequence of radiation injury and is characterized by increased infiltration of inflammatory cells (33), baseline SUVP90 could be an estimate of the degree of pretreatment lung inflammation and, therefore, possibly associated with increased susceptibility toward RP. In this study, we demonstrated that our RPEarly model can allow for patient-specific thoracic RT planning to minimize RPEarly and ICB discontinuation. Depending on the patient-specific pretreatment SUVP90, the same MLD is associated with a wide risk range of RPEarly. In addition, this study indicated that MLD can be further optimized by replanning the RT, which we did for a random 9-patient subset: We demonstrated that the original MLD could be reduced in all patients by 0.2–2.8 EQD23 Gy and from a median of 13 EQD23 Gy (range, 9–16 EQD23 Gy) to a median of 12 EQD23 Gy (range, 8–15 EQD23 Gy). This resulted in an RPEarly predicted risk reduction of 1%–6% and from a median of 15% (range, 8%–24%) based on the original MLD to 13% (range, 7%–21%) based on the replanned MLD. Reducing the rate of RPEarly beyond this would likely require more conformal treatment modalities including particle therapy.
This study provided critical information to guide RT planning and potential risk stratification for patients treated with cCRT and ICB consolidation and further highlighted ideal multidisciplinary expertise and management to optimize lung cancer care (34). Although this was a large study including 160 patients across a multiple-site center, the study had limitations such as a retrospective design based on patients with stage IIIA–IIIC locally advanced NSCLC, and no external validation was performed. These aspects encourage the need for rigorous validation (9) to assess model generalizability and thereby model applicability in other thoracic cohorts presenting with different disease, imaging, patient, and treatment characteristics.
CONCLUSION
Our findings demonstrate that in patients treated with cCRT and ICB consolidation, the RT dose to the normal lungs is strongly associated with the risk of RPEarly, the most consequential RP that is associated with poorer survival. Furthermore, we generated a risk model for RPEarly based on the upper end of SUV (SUVP90) of the normal lung from pretreatment 18F-FDG PET/CT together with the associated MLD. This model identifies the risk of RPEarly with higher accuracy than using MLD alone and could be used to enable patient-specific thoracic RTs specifically tailored to identify and mitigate the risk of RPEarly in the context of cCRT and ICB therapies. Such an approach would be anticipated to improve treatment tolerability and, thereby, decrease the likelihood of discontinuing ICB therapies and ultimately prolong survival.
DISCLOSURE
This research was funded in part through NIH/NCI Cancer Center support grant P30 CA008748. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does incorporating pretreatment 18F-FDG PET/CT features with RT dose yield models that would successfully identify patients with an exacerbated risk of RPEarly?
PERTINENT FINDINGS: A model was derived that combines the MLD of the normal lung with the SUVP90 of the normal lung. The model provided an improved ability to identify RPEarly risk over MLD alone.
IMPLICATIONS FOR PATIENT CARE: Incorporating pretreatment SUVP90 into clinical practice would enable the delivery of patient-specific RPEarly respecting RTs.
Footnotes
Published online Mar. 14, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 29, 2023.
- Revision received January 11, 2024.