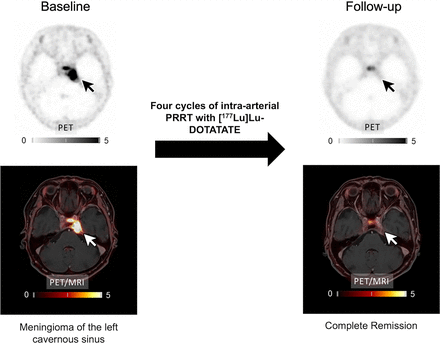
Visual Abstract
Abstract
Peptide receptor radionuclide therapy (PRRT) is a treatment option for patients with advanced meningioma. Recently, intraarterial application of the radiolabeled somatostatin receptor agonists has been introduced as an alternative to standard intravenous administration. In this study, we assessed the safety and efficacy of intraarterial PRRT in patients with advanced, progressive meningioma. Methods: Patients with advanced, progressive meningioma underwent intraarterial PRRT with [177Lu]Lu-HA-DOTATATE. The safety of PRRT was evaluated according to the Common Terminology Criteria for Adverse Events version 5.0. Treatment response was assessed according to the proposed Response Assessment in Neuro-Oncology criteria for meningiomas and somatostatin receptor–directed PET/CT. Results: Thirteen patients (8 women, 5 men; mean age, 65 ± 13 y) with advanced meningioma underwent 1–4 cycles (median, 4 cycles) of intraarterial PRRT with [177Lu]Lu-HA-DOTATATE (mean activity per cycle, 7,428 ± 237 MBq; range, 6,000–7,700 MBq). Treatment was well tolerated with mainly grade 1–2 hematologic toxicity. Ten of 13 patients showed radiologic disease control at follow-up after therapy (1/10 complete remission, 1/10 partial remission, 8/10 stable disease), and 9 of 13 patients showed good control of clinical symptoms. Conclusion: Intraarterial PRRT in patients with advanced meningioma is feasible and safe. It may result in improved radiologic and clinical disease control compared with intravenous PRRT. Further research to validate these initial findings and to investigate long-term outcomes is highly warranted.
Meningiomas are the most common primary neoplasms of the central nervous system (CNS) and account for more than one third of all cases (1). Meningiomas are mostly classified as benign (CNS World Health Organization [WHO] grade 1), but 10%–15% of cases are higher grade tumors that are considered atypical (CNS WHO grade 2) or even malignant or anaplastic (CNS WHO grade 3) (2).
The preferred treatment is complete surgical resection, when feasible, with external beam radiotherapy serving as a common alternative or adjunctive, depending on the WHO grade (3). However, 30%–40% of lesions are skull-base meningiomas that can be ineligible for surgery because of tumor localization in close proximity to critical intracranial structures such as the cavernous sinus or the optic nerve (4). Furthermore, recurrence rates are notably high, with 10%–30% of benign meningiomas and up to more than 80% of anaplastic meningiomas recurring within 5 y after surgery or external beam radiotherapy (5,6). Therefore, various pharmacologic treatment options have been developed, which, however, often show an increased toxicity and poor efficacy (7,8).
Peptide receptor radionuclide therapy (PRRT) with radiolabeled somatostatin receptor (SSTR) agonists such as [177Lu]Lu-DOTA-3-iodo-Tyr3-octreotate ([177Lu]Lu-high-affinity [HA]-DOTATATE) and [177Lu]Lu-(1,4,7,10-tetraazacyclododecane-N,N,N,N-tetraacetic acid)-Tyr3-octreotide ([177Lu]Lu-DOTATOC) is an established second-line therapy in the therapeutic regimen for neuroendocrine tumors of gastroenteropancreatic origin that typically overexpress SSTR (9).
Given that approximately 90% of meningiomas highly express SSTR, predominantly receptor subtype 2a (10), PRRT is a therapeutic option for patients with refractory or recurrent meningioma (11,12). Previous reports have demonstrated the general feasibility and good tolerability of SSTR-directed radionuclide therapy in meningioma (13–15). The most frequently observed adverse effects of intravenous PRRT include low to moderate nephrotoxicity, hepatotoxicity, and hematotoxicity with exception of a few cases of severe transient lymphocytopenia (11,13,16–20). In a more recent study, which included a long-term observation period of 5 y after combined external beam radiotherapy and PRRT, no relevant chronic adverse effects were found (15). Disease stabilization was largely achieved after PRRT, especially in patients with CNS WHO grade 1 or 2 meningioma (12,13).
Since most meningiomas are highly vascularized (21) and previous studies have shown that SSTRs are highly expressed not only on the tumor cell surface but also on the endothelium of the peritumoral vessels (22), intraarterial administration of PRRT might be a reasonable therapeutic approach to further enhance the therapeutic efficacy. In an initial case series, Vonken et al. observed a 2- to 4-fold increase in tumor uptake, and Braat et al. found an 11-fold increase through intraarterial administration of PRRT in comparison to intravenous procedures (23,24).
Most preliminary studies so far have investigated the safety of intraarterial PRRT in small patient cohorts during a short follow-up period. Reports of the radiologic and clinical outcome of intraarterial PRRT are largely limited as well, necessitating further investigations.
In this study, we assessed both the safety and efficacy of intraarterial PRRT in patients with advanced progressive meningioma during a long-term follow-up of up to 43 mo.
MATERIALS AND METHODS
Patients
Thirteen patients with locally advanced and progressive meningioma who were either ineligible for surgery, refractory to surgery or external beam radiotherapy, or declined surgery or external beam radiation therapy and underwent at least 1 cycle of intraarterially administered PRRT were included in this retrospective analysis. Patients with meningiomas located in close proximity to critical intracranial structures, such as the optic nerve, were included as well.
Each patient provided written informed consent following comprehensive medical information provided by a board-certified nuclear medicine physician. All procedures were performed in full compliance with the Declaration of Helsinki and its subsequent amendments and the legal considerations of clinical guidelines.
This study was approved by the Medical Ethics Committee of Ludwig-Maximilians-University of Munich, Munich, Germany (22-0907).
Preparations
Screening of patients included clinical assessment, laboratory tests, and 68Ga-DOTATOC PET/CT (SomaKit TOC; Advanced Accelerator Applications) to assess sufficient tracer accumulation according to current guidelines (with target lesion uptake significantly higher than the uptake of intracranial reference structures such as, for example, the sagittal sinus) (25). In all patients, cardiac MRI (performed 68 ± 16 d before PRRT) was available. Renal scintigraphy was performed to rule out advanced kidney impairment before therapy.
Administration of [177Lu]Lu-HA-DOTATATE
[177Lu]Lu-HA-DOTATATE (7,400 MBq/cycle; peptide mass, 150 μg; maximum 4 cycles) was applied directly into the tumor-feeding vessel as identified by diagnostic digital subtraction angiography. If multiple tumor-feeding vessels were present, a single, more proximal site of injection was chosen to cover all tumor-feeding vessels. A single injection position was preferred to avoid microcatheter manipulation and contamination of the angiography suite. In case of multifocal disease, the dominant lesion causing the most clinical symptoms was selectively treated (defined as the target lesion). During the fourth treatment cycle, the dominant artery feeding the tumor was embolized with precipitating hydrophobic injectable liquid 25%.
PRRT was performed according to the practical guidance on PRRT in patients with neuroendocrine tumors of the International Atomic Energy Agency, the European Association of Nuclear Medicine, and the Society of Nuclear Medicine and Molecular Imaging (26), with injection of an amino acid solution of lysine (2.5%) and arginine (2.5%) in combination with antiemetic drugs over a 4-h period during each treatment cycle to reduce renal retention of the radiopeptide.
Therapy Response
Assessment of treatment response included imaging (cardiac MRI and SSTR-directed PET/CT) as well as monitoring of clinical symptoms and laboratory parameters.
The radiologic response was determined on the target lesions according to the proposed Response Assessment in Neuro-Oncology criteria for meningiomas, using volumetric measurements on gadolinium-enhanced cardiac MRI (27).
Clinical response was assessed by frequent assessments (median time interval, every 2 mo) including a detailed patient history (focusing on tumor-related neurologic symptoms and common treatment-related side effects such as fatigue, asthenia, or alopecia) and physical examinations.
Toxicity
All patients underwent frequent laboratory testing (every 14 d), including renal function (creatinine, glomerular filtration rate), liver function (γ-glutamyl transferase, aspartate aminotransferase, alanine aminotransferase, albumin, international normalized ratio, total bilirubin), and blood count (hemoglobin, leukocyte count, platelet count). Clinical evaluations were performed between and during the treatment cycles. Safety was assessed according to the Common Terminology Criteria for Adverse Events version 5.0.
Statistical Analysis
Descriptive analysis was conducted including both qualitative and quantitative parameters. Quantitative parameters were assessed with consideration of mean, median, and SD.
RESULTS
Clinical Characteristics
Thirteen patients (8 women, 5 men; mean age, 65 ± 13 y) were included. The median time between primary diagnosis and the start of PRRT was 8 y (range, 0.5–20 y). All patients received SSTR-directed PET/CT (108 ± 12 MBq of [68Ga]Ga-DOTATOC, imaging 60 min after intravenous tracer administration) before PRRT to confirm receptor expression on the tumor cell surface. The target lesions showed a median SUVmax of 21.9 and a median SUVpeak of 14.5.
Eight patients had undergone at least 1 surgical intervention before PRRT, with 4 of them received additional external beam radiotherapy. Only 1 patient had undergone external beam radiotherapy alone, and 4 patients had not undergone any treatment before PRRT either because of inoperability of the tumor or because the patient rejected any other therapy. Detailed patient characteristics are outlined in Table 1.
Patient Characteristics*
Treatment Characteristics
Each patient underwent at least 1 cycle of intraarterially administered PRRT with a median of 4 cycles (range, 1–4 cycles; 4 patients had less than 4 cycles because of clinical deterioration, death, or the patient refused another treatment cycle). The median time interval between consecutive cycles was 9 wk (range, 6–13 wk). The technical success rate of angiography was 100%. On average, 7,428 ± 237 MBq of [177Lu]Lu-HA-DOTATATE was administered per cycle (range, 6,000–7,700 MBq). No dose reduction was required in any patient. The mean cumulative activity administered over all cycles was 25.7 ± 7.2 GBq of [177Lu]Lu-HA-DOTATATE (range, 7.5–30.1 GBq; detailed information on the administered activities can be found in Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org).
Safety
The treatment was generally well tolerated by all individuals. Immediately after PRRT or during the therapy-free interval, the most common adverse effects considered to be treatment-related were fatigue and asthenia. Two individuals (15.4%) suffered from severe nausea, which was attributable to the amino acid solution and subsided after completion of the infusion.
Other treatment-related adverse events included transient mild to severe headache (15.4%; 2/13) and minor alopecia (15.4%; 2/13).
All patients developed transient hematotoxicity at some point after therapy, including grade 3 anemia (7.7%; 1/13), thrombocytopenia (15.4%; 2/13), or lymphocytopenia (38.5%; 5/13), and 1 patient developed grade 4 lymphocytopenia (Supplemental Table 2). There were no cases of higher-grade hepatotoxicity or pathologic elevations of the international normalized ratio and total bilirubin in any patient (Supplemental Table 3).
One patient (7.7%) experienced transient grade 3–4 nephrotoxicity in the form of acute kidney injury of postrenal etiology, which was not directly attributable to PRRT (Supplemental Table 4; Supplemental Figs. 1–10). Another subject experienced progressive dizziness, facial paralysis, and painful limbs 3–6 mo after PRRT.
In patients with preexisting anemia or kidney impairment, there was no worsening of clinical condition during treatment, except for 1 patient who experienced first-stage chronic kidney failure before PRRT, who developed second-stage kidney failure at the end of follow-up.
One patient showed local necrosis and infection on a finger of the left hand, which might have been related to angiography. Apart from that, there were no severe angiography complications observed.
Efficacy
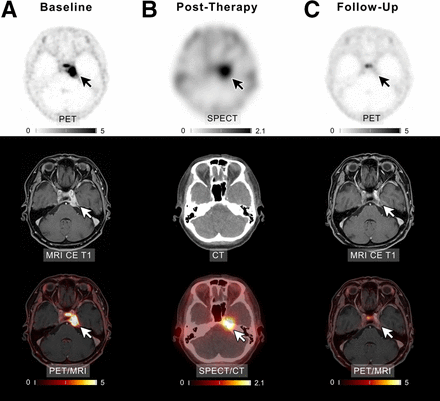
Radiologic treatment response was assessed according to the volumetric response criteria of the Response Assessment in Neuro-Oncology working group (27). At the end of treatment, 1 patient (7.7%) showed a complete remission (Fig. 1), 1 patient (7.7%) showed a partial remission, and 8 patients (61.5%) achieved stable disease. The pattern of tumor growth did not significantly change after therapy. Some larger tumors developed central necrosis, whereas smaller tumors diminished in size without altering their growth pattern. In cases in which the tumors remained stable in volume, no change in the growth pattern was observed. Follow-up imaging or end-of-therapy staging was not available in 3 patients because of either death or clinical deterioration.
Example of patient 3 with meningioma of unknown grade undergoing intraarterial PRRT with [177Lu]Lu-HA-DOTATATE. (A) Transaxial slices of baseline PET, contrast-enhanced (CE) T1-weighted MRI, and fused PET/MRI demonstrate SSTR-expressing meningioma in left cavernous sinus (white and black arrows). After 4 cycles of PRRT (posttherapeutic SPECT/CT imaging after the first cycle presented in B), complete remission according to RANO as well as PET criteria was recorded (C). In line with imaging, patient reported significant improvement of previous vertigo, headaches, and isolated unilateral abducens nerve palsy. Scale bars denote SUVs.
In terms of clinical outcome, 2 patients (15.4%) showed improvement of tumor-related symptoms, and 7 subjects (53.8%) were clinically stable. Two patients (15.4%) experienced clinical deterioration, and 1 of them passed away after the third treatment cycle because of progressive disease. Another patient died of heart failure, and another one was lost to follow-up and passed away more than a year later of unknown cause. Response assessment regarding clinical and radiologic outcome is shown in Table 2.
Outcome Including Imaging, Clinical Symptoms, and PFS
Progression-Free Survival (PFS) and Overall Survival (OS)
The median follow-up period was 24 mo (range, 1–43 mo). At the time point of censoring, median PFS in our cohort was 18 mo (CNS WHO grade 1, 24 mo; grade 2, 4 mo; unknown grading, 18 mo). The 6-mo and 12-mo PFS for the entire cohort was 76.9% (10/13; CNS WHO grade 1, 100%; grade 2, 25%; unknown grading, 100%).
Overall, 3 of 13 patients (23.1%) experienced either clinical (2 patients) or radiologic (1 patient) progression during the 24 person-years of follow-up. Three patients (23.1%) passed away 1 mo (patient 13), 8 mo (patient 8), and 14 mo (patient 5) after the first treatment cycle of heart failure, tumor progression, and unknown cause, respectively.
The 6-mo OS was 92.3% (12/13; CNS WHO grade 1, 100%; grade 2, 75%; unknown grading, 100%), and the 12-mo survival rate was 84.6% (11/13; CNS WHO grade 1, 100%; grade 2, 50%; unknown grading, 100%). Median OS has not been reached yet. The PFS and OS rates are listed in Supplemental Table 5.
DISCUSSION
As about 10%–30% of benign meningiomas and up to more than 80% of anaplastic meningiomas recur within 5 y after conventional therapy (5,6), PRRT offers a potential therapeutic option because of the high SSTR expression of the tumors (10–12). Given that meningiomas are often highly vascularized and SSTRs are also highly expressed on endothelial cells of peritumoral blood vessels (21,22), it appears that intraarterial administration of PRRT has the potential to deliver higher radiation doses to the tumor, which has been suggested in initial case series (23,24). In this study, we report a long-term follow-up of 13 meningioma patients who received a maximum of 4 treatment cycles of intraarterial PRRT.
Safety
To the best of our knowledge, this study is the first to investigate the toxicity and response to treatment with intraarterial PRRT with a long-term follow-up of up to 43 mo in a population of more than 10 patients. We were able to show that intraarterial administration of PRRT is well tolerated with only transient hemato-, hepato-, and nephrotoxicity being observed, except for higher-grade lymphocytopenia. Treatment-related clinical symptoms were transient and self-limiting in all cases.
Persistent deterioration of renal function occurred in 1 of our patients that experienced nephrotoxicity after PRRT (9.1%). In contrast, this rate was significantly higher (71%) in the previous study by Bodei et al. on intravenous PRRT in patients with neuroendocrine tumors (28). However, the comparability of the data is limited as the cohort of Bodei et al. was significantly larger than the one investigated in the current work (13 patients vs. 807 patients), and different tumor entities were analyzed. Furthermore, different radiopharmaceuticals had been used, and especially 90Y-labeled somatostatin analogs are known to be associated with higher renal toxicity rates (28). In a review by Mirian et al. on intravenous PRRT in patients with meningioma, the rate of permanent nephrotoxicity in all patients was even lower than that in our cohort (7.7% vs. < 1%) (11).
The patient with increasing dizziness, facial paralysis, and painful limbs 3–6 mo after PRRT had undergone surgery before PRRT, which led to postoperative bleeding. Considering the tumor location and radiologic tumor control, the reported symptoms seem to be more likely attributable to the postoperative complications rather than to PRRT or tumor progression. In comparison to a study on PRRT in patients with neuroendocrine tumors by Kobayashi et al. and to the NETTER-1 trial, the number of lymphocytopenia of any grade was higher in our patient cohort (85% vs. 50% vs. 18%) (16,19). However, higher-grade lymphocytopenia was comparable to the results reported by Kobayashi et al. (grade 3, 39% vs. 33%) but higher than in the NETTER-1 trial (grade 3–4, 38.5% vs. 9%) (16,19). Again, it should be noted that different tumor entities are compared here, and the number of participants of the prospective NETTER-1 trial is significantly larger than our patient cohort (16).
In comparison to the review by Mirian et al., the rate of higher-grade anemia, lymphocytopenia, and thrombocytopenia was higher among our patients (grade 3: anemia, 7.7% vs. <1%; lymphocytopenia, 38.5% vs. 11%; thrombocytopenia, 15.4% vs. 2%; grade 4: lymphocytopenia, 7.7% vs. <1%) (11). However, it needs to be considered that Mirian et al. analyzed a significantly higher number of patients (13 patients vs, 111 patients) of different studies with different radiopharmaceuticals being used (not only [177Lu]Lu-DOTATATE but also [177Lu]Lu-DOTATOC and [90Y]Y-DOTATOC) (11).
In contrast, a more recent study on intravenous PRRT in patients with meningioma by Minczeles et al. observed a higher rate of grade 3 and a comparable rate of grade 4 lymphocytopenia (grade 3: 38.5% vs. 53.3%; grade 4: 7.7% vs. 6.7%) (13). It is noteworthy that previous studies on PRRT in patients with neuroendocrine tumors demonstrated that high-grade therapy-associated lymphocytopenia did not lead to an increased incidence of severe infections (19,29).
One patient (7.7%) experienced local necrosis and infection on a finger of the left hand, possibly due to peripheral embolism after transfemoral catheterization, which is a rather rare complication of this procedure (30). There was no other possibly angiography-related complication observed.
Efficacy
In line with the existing literature on the results of intravenously administered PRRT (14,15,31,32), intraarterial PRRT also yielded a good therapeutic response in most of our patients. The disease control rate in our study was around 80%, with 1 patient achieving a complete remission and 1 patient a partial remission. Control of clinical symptoms was achieved in 9 of 13 patients (69%). In contrast, Minczeles et al. found a response rate of 40%, with no patient achieving a partial or complete remission (13). In comparison to results from Mirian et al., the rate of stable disease was slightly higher in our cohort (69% vs. 58%) as well as the rates of partial and complete remission (7.7% vs. 2% and 7.7% vs. 0%) (11). Again, the limited number of subjects enrolled in our study needs to be acknowledged.
Overall improvement of clinical symptoms was observed in 2 patients (15.4%). Two patients (15.4%) experienced clinical deterioration. One of these patients experienced dedifferentiation from CNS WHO grade 2 to grade 3 meningioma, which was histologically confirmed after surgery between 2 PRRT cycles. The other patient showed progressive frailty that could not be explained by imaging results. Since another surgical tumor resection had been performed in the meantime, the cause of the patient’s worsening condition could not be conclusively clarified.
One of our 13 patients (7.7%) experienced disease progression during treatment. Minczeles et al. reported a nearly 7-fold higher rate of progressive disease (53%) in their cohort, and the patients in the study by Mirian et al. showed a 5-fold higher rate (41%) (11,13).
According to the existing literature, patients with well-differentiated meningiomas appear to benefit more from PRRT (11). In their metaanalysis, Mirian et al. reported a 6-mo PFS rate of 94% for CNS WHO grade 1, 48% for CNS WHO grade 2, and 0% for CNS WHO grade 3 meningiomas. The 12-mo OS rates were 88%, 71%, and 52% (11). In comparison, the 6-mo PFS rate in our cohort was 100% in patients with CNS WHO grade 1 tumors, 25% in patients with CNS WHO grade 2 tumors, and 100% in patients with meningiomas of unknown grade. The 6-mo OS in our study was 100%, 75%, and 100% for patients with CNS WHO grade 1, grade 2, or unknown tumor grade, respectively, and the 12-mo OS was 100% (CNS WHO grade 1), 50% (CNS WHO grade 2), and 100% (unknown grading). At the time point of censoring, the median PFS was 18 mo, and the median OS has not yet been reached. In contrast, Bartolomei et al. reported a median PFS of 21 mo (32). However, it is noteworthy that their follow-up time ranged up to 77 mo, whereas our maximum follow-up time is limited to 43 mo, which could explain the lower median PFS in our cohort. Furthermore, 1 patient died from heart failure shortly after the first PRRT cycle, so she was not available for long-term follow-up.
In addition, intraarterially administered PRRT suggests promising efficacy compared with local therapy approaches such as surgical resection or external beam radiotherapy, with 6-mo PFS rates of 29% and a median OS of 10.6 mo (33).
Our findings suggest that intraarterial administration of PRRT may be a therapeutic option with favorable efficacy compared with local therapies and intravenous procedures. However, these are preliminary findings of a small patient cohort, and further prospective, multicenter studies are warranted.
Future Perspectives
In the future, individual dosimetry for each patient could further improve the results of intraarterial PRRT. Moreover, the therapeutic efficacy may be fostered using radiopharmaceuticals labeled with α-emitting radionuclides instead of the commonly used β-emitters. Another promising therapeutic option is the combination of external beam radiotherapy and intraarterial PRRT to further increase the achievable radiation doses to the tumor.
Limitations
The limitations of this study include its retrospective design and the limited number of patients with heterogeneous previous treatments. Furthermore, most subjects received additional tumor embolization during the final treatment cycle, which may have led to an additional therapeutic effect. Currently, dosimetry data are not yet available for this patient cohort. Therefore, an analysis to this effect is planned as a future project to evaluate whether higher tumor uptake is associated with a better therapeutic efficacy. Despite these limitations, our study provides valuable information on the feasibility, tolerability, and efficacy of intraarterial administration of PRRT in patients with advanced progressive meningioma, a subject for which the existing literature is rather limited.
CONCLUSION
To the best of our knowledge, this retrospective analysis is the first to investigate the long-term toxicity and efficacy of intraarterially administered PRRT in a cohort of more than 10 patients with progressive meningioma. Intraarterial PRRT is feasible and safe without significant additional toxicity and can lead to better radiologic and clinical disease control compared with intravenous PRRT. Further research to validate these initial findings and to investigate long-term outcomes is highly warranted.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is the intraarterial administration of PRRT a feasible and safe therapeutic option with a superior therapeutic efficacy compared with intravenous PRRT for patients with meningioma?
PERTINENT FINDINGS: In our retrospective study, intraarterial PRRT was well tolerated in all individuals and showed good therapeutic efficacy. No chronic adverse effects were observed.
IMPLICATIONS FOR PATIENT CARE: Intraarterial PRRT is feasible and safe with no additional toxicity compared with intravenous PRRT, and it may lead to better disease control rates than intravenous procedures; however, further research to validate these initial findings is highly warranted.
Footnotes
Published online Oct. 24, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication June 9, 2024.
- Accepted for publication September 19, 2024.