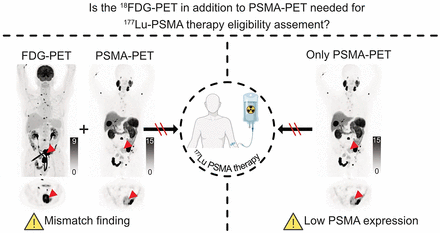
Visual Abstract
Abstract
18F-FDG and prostate-specific membrane antigen (PSMA) PET have been used to assess eligibility for PSMA-targeted therapy by some centers. However, it remains unclear whether both examinations are needed as a part of workup in the clinical practice or whether PSMA PET alone, as was done in the positive phase 3 VISION trial, is sufficient to identify suitable candidates. The aim was to reanalyze all patients who underwent both 18F-FDG and PSMA PET for PSMA-targeted therapy eligibility assessment using the VISION trial criteria. Methods: Eighty-nine men with metastatic castration-resistant prostate cancer referred to 177Lu-PSMA therapy from June 2019 to October 2021 who underwent both 18F-FDG and PSMA PET (using either 68Ga-PSMA-11 or 18F-PSMA-1007) examinations within 2 wk were included in this analysis. Eligibility status was determined in accordance with either knowledge of both 18F-FDG and PSMA PET (clinical routine) or VISION criteria with PSMA PET–only (study reassessment, done twice with liver only for PSMA-11 and liver/spleen as reference for PSMA-1007). A metastasis seen on 18F-FDG PET or CT but not on PSMA PET was denoted as a mismatch finding and led to exclusion from 177Lu-PSMA therapy. On the basis of clinical assessment, 52 patients received 177Lu-PSMA therapy, and 37 did not; all patients were reassessed. Results: Patients treated with 177Lu-PSMA therapy had significantly longer overall survival than those not treated (12.4 vs. 6.8 mo, P < 0.01). PSMA-only analysis (spleen/liver reference) and 18F-FDG/PSMA mismatch reads had substantial agreement (Cohen κ = 0.73). Eighteen percent (n = 16/89) of patients had a mismatch finding based on 18F-FDG/PSMA PET. With the liver/spleen reference, a minor fraction of patients who had no mismatch finding (and were therefore treated) would have been withheld from therapy by PSMA-only analysis (3%). Three percent (n = 3) of all patients had an 18F-FDG/PSMA mismatch finding not detected by PSMA PET–only (VISION-like) analysis. For patients not receiving PSMA therapy, the overall survival was not statistically significantly different comparing 18F-FDG/PSMA mismatch versus nonmismatch (P = 0.61) patients. Conclusion: 18F-FDG and PSMA PET provide complementary information, yet less than 5% of patients had mismatch findings not detected using PSMA PET–only. Based on our data, 18F-FDG/PSMA mismatch examination and PSMA-only analysis have a substantial level of agreement.
Radioligand therapy targeting the prostate-specific membrane antigen (PSMA) with 177Lu (177Lu-PSMA) is an efficacious therapy option in patients with end-stage metastatic castration-resistant prostate cancer (1). Recently, the VISION trial, an open-label international phase 3 trial comparing PSMA therapy against standard of care, demonstrated superiority of the additional 177Lu-PSMA therapy compared with standard of care only; overall survival was significantly longer when receiving 177Lu-PSMA therapy with standard of care (2). This led to U.S. Food and Drug Administration approval in March 2022. This approval is a hallmark for nuclear medicine, as it is the first novel theragnostic treatment option available for an entity with high prevalence (in contrast to relatively rare neuroendocrine tumors).
Men with metastatic castration-resistant prostate cancer have multiple treatment options available, and 177Lu-PSMA is now being tested in earlier treatment lines (3,4). Identification of patients who are most suited for PSMA therapy is critical for outcome, given the rate of nonresponders of approximately 50% (RECIST response in the VISION trial) (2). This is relevant, because pretherapeutic PSMA PET should allow for an improved prognostication of overall survival time and prediction of response, as it directly assesses the expression of the PSMA target (5,6). To assess eligibility, the VISION trial relied on PSMA PET in combination with diagnostic CT to exclude patients with low PSMA expression in metastases that meet specific size criteria (7). Patients not fulfilling the criteria had worse overall survival, which was shown by a subsequent analysis (8). The use of PSMA PET–only to assess 177Lu-PSMA therapy eligibility was adopted by many departments of nuclear medicine and is considered the clinical standard (9).
In contrast, the initial prospective 177Lu-PSMA therapy trials used both PSMA and 18F-FDG PET examinations to assess therapy eligibility; this procedure was adopted by many departments of nuclear medicine, including ours (10,11). Dual tracer screening was implemented assuming that a PSMA-negative metastasis that is missed by PSMA PET might heavily influence the response to 177Lu-PSMA therapy. An 18F-FDG–positive and PSMA-negative metastasis is denoted as a mismatch finding.
However, it remains unclear whether the combination of PSMA and 18F-FDG is clinically needed. Therefore, the aim of this study was to compare 18F-FDG/PSMA mismatch evaluation head-to-head with an analysis relying only on PSMA PET. To this end, we performed a retrospective reread of the pretherapeutic PSMA PET images according to the VISION trial protocol.
MATERIALS AND METHODS
Patient Cohort
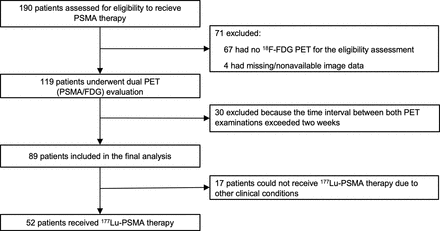
Among 119 patients who were referred to PSMA and 18F-FDG PET to assess 177Lu-PSMA therapy eligibility at the University Hospital Essen between June 2019 and October 2021, the patients whose image data were available and whose 18F-FDG and PSMA PET images were obtained within 2 wk of each other (n = 89) were included. Patient characteristics are shown in Table 1. A total number of 52 patients were treated with 177Lu-PSMA therapy, whereas 37 patients were not treated with 177Lu-PSMA therapy. Median prostate-specific antigen (PSA) level was 176 ng/mL (interquartile range, 32.5–526.3) in the cohort receiving 177Lu-PSMA therapy and 65.7 ng/mL (interquartile range, 16.8–290.7) in the remaining patients. In total, 53 (59.6%) patients underwent PSMA-11 PET, whereas 36 (40.4%) patients underwent PSMA-1007 PET examination. Ethical approval for this retrospective study was present (local ethics committee approval no. 19-8570-BO).
Patient Characteristics
Clinical 18F-FDG/PSMA Mismatch Analysis to Assess Therapy Eligibility
Patients with PSMA and 18F-FDG PET for PSMA therapy assessment with a maximum interval of 2 wk between the PET examinations were analyzed. In our clinical routine, 177Lu-PSMA therapy eligibility was assessed based on visual analysis of PSMA PET and 18F-FDG PET to rule out clinically relevant mismatch. Inspired by the target lesion definition of the RECIST 1.1 criteria, visceral metastases/soft-tissue lesions with longest diameter of at least 10 mm and lymph nodes with short-axis diameter exceeding 15 mm that have 18F-FDG uptake higher than liver and PSMA uptake lower than spleen/liver were considered as clinically relevant mismatches. In addition, for the bone lesions, more than 3 bone metastases without osteolytic correlates, which are regarded as unmeasurable in RECIST 1.1 criteria, with 18F-FDG uptake higher than liver and PSMA uptake lower than liver was regarded as a clinically relevant mismatch (12). Visual uptake generally higher than liver or spleen for all lesions on PSMA PET was necessary for therapy eligibility. All men were discussed in a multidisciplinary tumor board. A metastasis in organs or bone delineated on 18F-FDG PET with no corresponding PSMA uptake was rated as a mismatch finding, and the patient was excluded from 177Lu-PSMA therapy. The mean activities administered for 68Ga-PSMA-11 and 18F-PSMA-1007 PET were 117.5 ± 56.5 and 328.3 ± 76.3 MBq, respectively. Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org) provides details on the criteria used to assess 177Lu-PSMA therapy eligibility. Clinical reads of PET images have been reassessed by 2 nuclear medicine physicians to ensure consistency.
Retrospective Application of the VISION PSMA PET–Only Eligibility Criteria
All PSMA PET examinations were analyzed by the same nuclear radiologist who helped design the criteria, trained the readers, and supervised the centralized eligibility analysis for the VISION trial. The reader was unaware of the clinical assessment and 18F-FDG PET acquisition. If available, diagnostic contrast-enhanced CT was used as was done for the VISION trial, and if not available, the in-line CT from the PET/CT was used. Images were viewed using MIM Software 7.1.7. Analysis was completed twice and in accordance with VISION criteria, which only used PSMA-11; first, the liver was regarded as a reference organ for positivity threshold. In a second approach, for patients who were imaged with PSMA-1007 and excluded because of low PSMA expression, the spleen was used as a reference organ. In summary, to be VISION eligible, PSMA-positive lesions above the organ threshold (liver or spleen) and no PSMA-negative lesion had to be present; to ensure the latter, the CT component was used. PSMA negativity of the following CT findings meeting these size criteria led to exclusion: lymph node of at least 2.5 cm; solid organ metastases of at least 1-cm short axis; bone metastases with soft-tissue component of at least 1 cm.
PSMA Therapy
Besides the previously described image-based criteria for therapy eligibility, the EANM procedure guidelines were followed (9). 177Lu-PSMA therapy was performed as previous published (13). Briefly, the PSMA-617 ligand (ABX GmbH) was conjugated with 177Lu (ITG Isotope Technology). A median cumulative dose of 24.4 (interquartile range, 12.3–29.8) GBq was administered per patient; cycles were repeated every 6–8 wk.
Statistical Analysis
R and SPSS (Version 29; IBM) were used for statistical analysis, testing, and plotting. Kaplan–Meier estimates were used. Cox regression analysis was used for analysis of censored data, and the log rank test was used to compare groups regarding survival time. Agreement between PSMA-only analysis (using spleen/liver as a reference organ) and 18F-FDG/PSMA mismatch read was evaluated with Cohen κ analysis. Difference in PSA response rate of at least 50% for the patients treated with 177Lu-PSMA was analyzed with a χ2 test. P < 0.05 was regarded as statistically significant.
RESULTS
Detection of 18F-FDG/PSMA Mismatch Using PSMA PET Alone
Eighty-nine of 119 patients referred to PSMA therapy underwent 18F-FDG and PSMA PET within 2 wk of each other (Fig. 1). The rate of 18F-FDG/PSMA mismatch findings was 18% (n = 16/89). Substantial agreement between PSMA-only analysis (in accordance to modified VISION criteria using liver/spleen as a reference organ) and 18F-FDG/PSMA mismatch read was observed (n = 81/89, 91%, Cohen κ: 0.73; Fig. 2). Three percent (n = 3/89, denominator: total cohort) had an 18F-FDG/PSMA mismatch finding, although they were deemed eligible for PSMA therapy by the PSMA-only analysis (Fig. 3; Table 2). Twelve percent (n = 11/89, denominator: total cohort) had no mismatch finding and were not eligible for 177Lu-PSMA therapy according to the VISION-like analysis (of this group, not all patients were treated with PSMA therapy because of insufficient clinical parameters).
Flow chart of included patients.
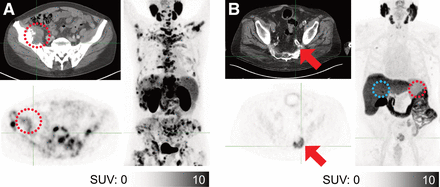
Exemplary patient who was ineligible for 177Lu-PSMA therapy (PSMA-only evaluation and 18F-FDG/PSMA assessment). Large osteolytic lesion in the sacrum with soft-tissue component (A, yellow arrow) with low PSMA uptake (B, red arrows) and intensive 18F-FDG uptake (C, blue arrows). 18F-FDG has also shown additional liver metastases that were not detected by non–contrast-enhanced CT or PSMA PET (C, arrowheads).
Exemplary patient who showed 18F-FDG/PSMA mismatch that was not detected by PSMA-only analysis. PSMA-11 PET/CT showed no significant CT correlate of bone lesions (A), which have high PSMA uptake (B). However, 18F-FDG PET/CT showed more than 20 additional bone lesions (C).
Differences Between 177Lu-PSMA Eligibility Decisions Made by Our Department (Using 18F-FDG and PSMA PET) and PSMA-Only (VISION-like) Reevaluation
Of the 89 analyzed patients referred to PSMA therapy, 52 patients (58%) received PSMA therapy. Table 2 provides details of the reasons for exclusion from 177Lu-PSMA therapy. Of those patients treated, 7 patients (13%, n = 7/52, denominator: treated patients) were treated because of the clinical assessment but would not have been eligible for 177Lu-PSMA therapy according to the PSMA-only (VISION-like) analysis. Of patients treated with 177Lu-PSMA therapy, 23 patients (44%, n = 23/52, denominator: treated patients) had undergone PSMA-1007 PET for eligibility assessment.
VISION-like Analysis of Patients (Separated According to the PSMA Ligand Used)
This first assessment used the VISION-prescribed threshold of activity greater than liver for PSMA positivity and likewise activity equal to or less than liver for PSMA negativity. In the cohort imaged with PSMA-11, 3 patients (6%, n = 3/53, denominator: patients imaged with PSMA-11) deemed eligible by the PSMA-only analysis would have been ineligible because of 18F-FDG/PSMA mismatch findings. In the PSMA-1007 cohort, no patient with a mismatch finding was rated as therapy eligible by the PSMA-only analysis. Only 1 treated patient (2%, n = 1/53, denominator: patients imaged with PSMA-11) without an 18F-FDG/PSMA mismatch finding was excluded in the PSMA-11 cohort though the VISION read. However, 6 treated patients imaged with PSMA-1007 (17%, n = 6/36, denominator: patients imaged with PSMA-1007) were excluded from PSMA therapy based on the PSMA-only read without a mismatch finding.
VISION-like Analysis of Patients with Adjusted Reference Organ
To adjust for the higher hepatic PSMA uptake, the eligibility of patients imaged with PSMA-1007 was reassessed using the spleen as additional reference organ (Fig. 4). After this adjustment, only 3 patients (3%, n = 3/89, denominator: total cohort) of the total cohort including patients imaged with either PSMA tracer were not eligible because of the PSMA-only VISION analysis but showed no mismatch finding and were treated. For PSMA-1007, only 2 patients (6%, n = 2/36, denominator: patients imaged with PSMA-1007) were excluded without a mismatch finding and were treated.
Exemplary cases of patients referred to 177Lu-PSMA therapy. PSMA-11 PET imaging showed destructive osseous metastasis with large PSMA-negative soft-tissue component (A, dashed red circle). Therefore, patient was rated as not eligible for 177Lu-PSMA therapy by PSMA PET–only VISION analysis. PSMA-1007 PET imaging demonstrates bone metastasis (B, arrow) with uptake lower than liver (B, blue dashed circle), and thus VISION analysis excluded the patient from 177Lu-PSMA therapy. In the modified VISION analysis using spleen instead of liver as threshold organ, the patient was included as bone metastasis had higher uptake than spleen (B, red dashed circle).
However, only 3 patients (3%, n = 3/89, denominator: total cohort) of the total cohort had a mismatch finding, which was not detected by the PSMA-only analysis (Table 2). See supplemental Table 2 for details on mismatch and PSMA-only VISION evaluation deviations. For comparison, supplemental Figure 1 provides the cross tables for the clinical reads (mismatch finding or low PSMA expression) and the VISION analysis (original and spleen adjusted) separately for the used ligand.
Overall Survival of Total Cohort and Untreated Patients
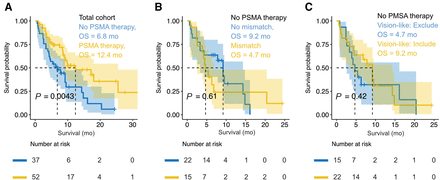
The overall survival of patients treated with 177Lu-PSMA therapy was significantly longer than that of those not treated (12.4 [95% CI, 8.6–25.5] vs. 6.8 [95% CI, 4.2–9.5] mo, P < 0.01; hazard ratio, 0.454, P < 0.01).
The overall survival of patients not treated with 177Lu-PSMA therapy was not statistically significantly different between those with (n = 15) or without an 18F-FDG/PSMA mismatch (n = 22) finding (4.7 [95% CI, 2.4–6.8] vs. 9.2 [95% CI, 3.3–14.3] mo, P = 0.61; hazard ratio, 1.224, P = 0.6), but this analysis was limited because of the low number of patients (n = 37). Patients not treated with 177Lu-PSMA therapy did not have a statistically significantly different survival time if they were VISION (spleen adjusted) eligible or not (4.7 [95% CI, 2.4–16.1] vs. 9.2 [95% CI, 3.3–14.3] mo, P = 0.42; hazard ratio, 0.73, P = 0.4; Fig. 5).
Overall survival of entire cohort and patients not treated with 177Lu-PSMA therapy. Overall survival for total cohort of patients with appropriate PET examinations is shown (A); patients treated with 177Lu-PSMA therapy have significantly longer overall survival time. Looking at patients who were not treated with 177Lu-PSMA therapy, there was no statistically significant difference in patients with a mismatch finding compared with those without (B). Likewise, patients excluded from 177Lu-PSMA therapy according to the PSMA-only VISION evaluation (spleen-adjusted threshold for PSMA-1007 group) did not have shorter survival compared with excluded patients (C).
Outcome of the Patients Receiving 177Lu-PSMA
Of the 89 analyzed patients referred to our department, 52 patients (58%) received 177Lu-PSMA therapy. PSA50RR of all patients treated with 177Lu-PSMA was 51%. Of those patients treated, 7 patients would not have been eligible for 177Lu-PSMA therapy according to the PSMA-only (VISION-like, only liver used as reference) analysis but were still treated because of the clinical assessment. PSA50RR of those patients was not statistically significantly different from patients who were eligible (40% vs. 52.4%, P = 0.66). The overall survival time of patients who were clinically treated with 177Lu-PSMA, although they should have been excluded according to VISION reevaluation, was 7.46 mo (n = 7; 95% CI, 5.2–18.3) in contrast to 12.4 mo (95% CI, 4.7–20.1) of the patients who were eligible and treated with 177Lu-PSMA; the difference was not statistically significant (P = 0.7).
DISCUSSION
In the present study, we investigated different imaging approaches to assess eligibility for 177Lu-PSMA therapy and found high agreement of PSMA-only and 18F-FDG/PSMA mismatch assessment. Specifically, we explored the need for 18F-FDG PET in addition to PSMA PET. Only 3% of patients were deemed ineligible for therapy in excess of a PSMA-only analysis because of 18F-FDG/PSMA mismatch findings on 18F-FDG and PSMA PET. Seven patients (n = 7/89; 8%, denominator: total cohort) were excluded because of the PSMA-only VISION-like analysis but clinically treated with PSMA therapy, and this was only 3 patients (3%) if the reader used the PSMA-only modified VISION criteria with liver as the reference organ for PSMA-11 and spleen for PSMA-1007.
177Lu-PSMA therapy is an emerging treatment option in prostate cancer, which builds on the theragnostics principle, meaning that the diagnostic target can be used for whole-body imaging and therapeutic approaches (14). The assessment of PSMA expression is therefore a prerequisite to assess therapeutic eligibility (15). However, the rate of nonresponders is considerably high, motivating the search for additional selective examinations before 177Lu-PSMA therapy. The reason for this lies in the tumor biology of advanced prostate cancer. Prostate cancer has a remarkable early tendency to spread to distant organs; at the time of prostatectomy, up to 70% of patients have prostate cancer cells in the bone marrow (16). This may lead to a parallel progression of distinct cancer phenotypes and dedifferentiation throughout the course of the disease, leading to tumor heterogeneity (17). In fact, neuroendocrine transdifferentiation may lead to loss of PSMA expression and often occurs in liver metastases (18). Therefore, liver metastases are associated with worse overall survival rate and require dedicated treatment, especially when 177Lu-PSMA therapy is used; otherwise, transdifferentiated metastases without PSMA expression would not be adequately targeted (19,20).
The assessment of tumor heterogeneity of advanced prostate cancer is challenging (21). Using PSMA PET alone, distinct uptake patterns can be observed that are associated with distinct rates of overall survival (22). Especially, low PSMA expression is associated with short overall survival time (6,22,23). The PSMA expression is also relevant to assess the PSMA tumor volume response to systemic therapy; otherwise, decreasing PSMA tumor volume can be erroneously assessed as response to therapy, which could also represent a reduction of differentiated tumor volume with an increase of dedifferentiated proportions (24). To this end, PSMA/18F-FDG mismatch examination may be used; multitracer approaches may reveal considerable tumor heterogeneity in prostate cancer, especially in end-stage prostate cancer under PSMA therapy (25,26).
We have found a rate of patients with mismatch findings of 18%, which is in line with previous reports (27). Interestingly, the overall survival rate of patients who were not treated with PSMA therapy was not different comparing patients with and without mismatch finding (4.7 vs. 9.2 mo, P = 0.61). However, a tendency to shorter overall survival in case of mismatch is recognizable in the cohort of patients who did not receive 177Lu-PSMA therapy. This could indicate that the tumor phenotype may not be adequately characterized by manual mismatch analysis (i.e., searching for metastases with a flip-flop phenomenon). We have presented the characteristics of patients who have not received PSMA therapy in Supplemental Table 3 for those with and without a mismatch finding. There was no difference regarding the levels of PSA, lactate dehydrogenase, alkaline phosphatase, or hemoglobin. However, a confounding effect could still be present, causing the mismatch and nonmismatch groups to have a similar overall survival by disguising a potential difference. Also, the finding might partially be explained by the definition of mismatch; patients rated as mismatch could potentially also show less PSMA uptake and would therefore have been excluded from therapy in a PSMA-only VISION analysis. In contrast, Michalski et al. (28) showed that patients receiving 177Lu-PSMA therapy have a significantly shorter overall survival in case of a mismatch finding. This could be in line with our finding because we compared the implications of mismatch in a cohort not treated with PSMA therapy; therefore, the lower PSMA expression of patients with mismatch was not linked to treatment efficacy. However, the potential value of 18F-FDG PET before PSMA therapy start might be in assessing the prognosis of the patient. Recently, it was shown that PSMA PET was predicting response to PSMA therapy, whereas 18F-FDG PET was prognosticating the outcome (29). Therefore, 18F-FDG PET might have a valuable role in addition to the mismatch assessment.
In contrast to previous phase 2 trials, we did not require a specific SUV threshold for therapy eligibility but used visual uptake higher than liver (10,11). The TheraP study and earlier LuPSMA trial required higher PSMA positivity for eligibility (SUVmax of 20 in 1 lesion and of 10 in remaining lesions or SUVmax higher than 1.5 times liver activity) (10,21). This higher threshold may select for patients who respond better to 177Lu-PSMA but also withhold therapy from many patients who would have benefited. We found that the liver as the reference organ for PSMA-1007 may lead to the exclusion of patients who were clinically treated with 177Lu-PSMA therapy. Therefore, we proposed the spleen as an alternative reference organ for patients imaged with PSMA-1007 before 177Lu-PSMA therapy, which is in line with previous publications. For example, the spleen was recently recommended as a reference organ for the PROMISE framework (miTNM criteria) instead of the liver for PSMA ligands with liver dominant excretion (30). Also, the spleen was used as reference in a study comparing PSMA-11 and PSMA-1007 (31).
CONCLUSION
The combination of 18F-FDG and PSMA PET may help in the assessment of tumor heterogeneity and dedifferentiation in end-stage prostate cancer, yet only a small fraction of patients was withheld from therapy because of 18F-FDG/PSMA mismatch findings not detected by PSMA-only VISION analysis. Further studies investigating the potential of 18F-FDG/PSMA imaging for predicting treatment response to 177Lu-PSMA therapy are warranted.
DISCLOSURE
Phillip Kuo is a consultant or speaker for Amgen, Bayer, Chimerix, Eisai, Fusion Pharma, General Electric Health Care, Invicro (also prior employee), Novartis, and UroToday. He is a recipient of research grants from Blue Earth Diagnostics and General Electric Health Care. Robert Seifert has received research funding from the Else Kröner-Fresenius-Stiftung and from Boehringer Ingelheim Fonds. Wolfgang Fendler reports fees from SOFIE Bioscience (research funding), Janssen (consultant, speakers bureau), Calyx (consultant), Bayer (consultant, speakers bureau, research funding), Parexel (image review), and AAA (speakers bureau) outside of the submitted work. Ken Herrmann received personal fees from BTG, Bayer, Sofie Biosciences, SIRTEX, Adacap, Curium, Endocyte, IPSEN, Siemens Healthineers, GE Healthcare, Amgen, Novartis, ymabs, Aktis, Oncology, and Pharma15, as well as nonfinancial support from ABX and grants from BTG. Boris Hadaschik has had advisory roles for ABX, AAA/Novartis, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen R&D, Lightpoint Medical, Inc., and Pfizer; has received research funding from Astellas, Bristol Myers Squibb, AAA/Novartis, German Research Foundation, Janssen R&D, and Pfizer and has received compensation for travel from Astellas, AstraZeneca, Bayer, and Janssen R&D. Tugce Telli received support from the German Academic Exchange Service. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 18F-FDG PET needed to assess 177Lu-PSMA therapy eligibility?
PERTINENT FINDINGS: The VISION-like analysis, which only regarded PSMA PET and CT to assess eligibility for 177Lu-PSMA therapy, resulted in a minor rate of patients who showed an 18F-FDG/PSMA mismatch finding that has been not detected; therefore, the mismatch evaluation before the start of PSMA therapy might be omitted. A spleen-adjusted threshold should be used for PSMA-1007 imaging studies to assess therapy eligibility.
IMPLICATION FOR PATIENT CARE: With careful evaluation, PSMA PET/CT alone might be sufficient for 177Lu-PSMA therapy eligibility assessment. However, further studies investigating the potential of 18F-FDG/PSMA for outcome prognosticating of patients treated with 177Lu-PSMA therapy are warranted.
Footnotes
Published online Dec. 15, 2022.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication August 1, 2022.
- Revision received December 6, 2022.