Abstract
Currently, biomarkers that predict the efficacy of everolimus in metastatic renal cell carcinoma (mRCC) patients are lacking. Everolimus inhibits vascular endothelial growth factor A (VEGF-A) expression. We performed PET scans on mRCC patients with 89Zr-bevacizumab, a VEGF-A–binding antibody tracer. The aims were to determine a change in tumor tracer uptake after the start of everolimus and to explore whether 89Zr-bevacizumab PET can identify patients with early disease progression. Methods: 89Zr-bevacizumab PET was done before and 2 and 6 wk after the start of everolimus, 10 mg/d, in mRCC patients. Routine CT scans were performed at baseline and every 3 mo thereafter. Tumor tracer uptake was quantified using SUVmax. The endpoints were a change in tumor tracer uptake and treatment response on CT after 3 mo. Results: Thirteen patients participated. The median SUVmax of 94 tumor lesions was 7.3 (range, 1.6–59.5). Between patients, median tumor SUVmax varied up to 8-fold. After 2 wk, median SUVmax was 6.3 (1.7–62.3), corresponding to a mean decrease of 9.1% (P < 0.0001). Three patients discontinued everolimus early. At 6 wk, a mean decrease in SUVmax of 23.4% compared with baseline was found in 70 evaluable lesions of 10 patients, with a median SUVmax of 5.4 (1.1–49.4, P < 0.0001). All 10 patients who continued treatment had stable disease at 3 mo. Conclusion: Everolimus decreases 89Zr-bevacizumab tumor uptake. Further studies are warranted to evaluate the predictive value of 89Zr-bevacizumab PET for everolimus antitumor efficacy.
Clear cell renal cell carcinoma (RCC) is characterized by Von Hippel–Lindau gene inactivation. This results in expression of proangiogenic growth factors such as vascular endothelial growth factor A (VEGF-A) and typical vascular tumors. Patients with RCC have higher circulating VEGF-A levels than healthy controls (1). A high baseline plasma VEGF-A level is associated with shorter progression-free survival (PFS) and overall survival (OS) in metastatic RCC (mRCC) and is an independent prognostic factor (2). VEGF-A pathway–targeting agents such as the VEGF-A–binding antibody bevacizumab with interferon alfa or the tyrosine kinase inhibitors sunitinib, sorafenib, pazopanib, and axitinib are established treatment options for mRCC (3–6). Inhibitors of mammalian target of rapamycin (mTOR) also have antitumor activity in mRCC (7,8). mTOR plays a key role in cell growth, protein translation, autophagy, and metabolism. Blocking mTOR causes cell cycle arrest in a broad range of tumor types, but in addition to a direct antitumor effect, mTOR inhibitors block VEGF-A expression, vascular permeability, endothelial cell proliferation, and angiogenesis (9).
Everolimus is an orally administered mTOR inhibitor. A phase III study in mRCC patients with progressive disease during or after treatment with sunitinib, sorafenib, or both demonstrated doubling of median PFS for everolimus compared with placebo (4.9 mo vs. 1.9 mo) (10). Recently, nivolumab was proven to be superior to everolimus in pretreated mRCC patients, with median OS of 25.0 mo compared with 19.6 mo, and fewer high-grade, treatment-related adverse events (11).
Only a subset of mRCC patients profits from treatment with mTOR inhibitors. Regretfully, no predictive biomarkers that can identify patients who are likely to benefit from mTOR inhibitors are available. The novel treatment option with the immunotherapeutic drug nivolumab after first-line antiangiogenic treatment further reinforces the need for such a predictive biomarker. 89Zr-bevacizumab is a PET tracer that binds VEGF-A. We demonstrated in a previous study that 89Zr-bevacizumab PET scans can visualize RCC tumor lesions and that tracer uptake in these lesions changed during treatment with sunitinib or bevacizumab/interferon alfa. Furthermore, results suggested that high baseline tumor uptake was associated with a longer time to progression (12). Serial 89Zr-bevacizumab PET scans might also be useful to assess downregulation of tumor VEGF-A expression after the start of an mTOR inhibitor and serve as an early indicator of efficacy. We hypothesize that successful downregulation of VEGF-A expression by everolimus results in a decrease in tumor tracer uptake after the start of treatment.
We conducted a feasibility study in patients with mRCC who started treatment with everolimus. The primary objective of this study was to demonstrate a change in tumor tracer uptake after the start of treatment. The secondary objective was to explore whether 89Zr-bevacizumab PET could identify patients with disease progression on the first follow-up CT scan after 3 mo of treatment.
MATERIALS AND METHODS
Patients
Adult mRCC patients who had measurable disease, had a World Health Organization performance score of 2 or less, and were candidates to start treatment with everolimus were eligible. The study was approved by the institutional review board, and all subjects gave written informed consent. The trial is registered on ClinicalTrials.gov (NCT01028638).
Study Design and Treatment
Patients underwent 89Zr-bevacizumab PET imaging before and 2 and 6 wk after the start of everolimus, 10 mg orally once daily. Treatment was continued until disease progression or unacceptable toxicity. The primary endpoint was a change in 89Zr-bevacizumab uptake in tumor lesions between the baseline PET scan and the scans after 2 and 6 wk of treatment. The secondary endpoint was progressive disease according to RECIST 1.1 after 3 mo of treatment.
Imaging Techniques
Four days before each PET scan, the patients were injected intravenously with 37 MBq of 89Zr-bevacizumab, protein dose 5 mg. No therapeutic effects are to be expected from this dose because therapeutic bevacizumab doses vary from 7.5 to 15 mg per kilogram of body weight. Conjugation and labeling were done as described before (13). The patients were scanned from head to upper thigh in up to 8 consecutive bed positions as described earlier (12), with a final reconstruction resolution of about 10 mm.
At baseline and every 3 mo during treatment, the patients underwent CT imaging. CT was performed with oral and intravenous contrast and a maximal slice thickness of 5.0 mm. The mean interval between the baseline CT and baseline PET scans was 15 d.
Imaging Data Analysis
The baseline PET scans were fused with the baseline CT scans and analyzed by visual and quantitative assessment of tumor lesions. All hot spots on PET that were concordant with tumor lesions on CT were documented as being metastases. For reliable quantification of 89Zr-bevacizumab uptake, lesions had to be larger than 10 voxels and had to be delineable from normal-organ background. Irradiated lesions were excluded from quantification. Quantification was performed with AMIDE Medical Image Data Examiner software (version 0.9.1; Stanford University) (14). SUVmax and SUVmean were calculated for tumor lesions and healthy organs. SUVmax and SUVmean correlated strongly (r = 0.99, P < 0.0001) (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). SUVmax is less operator-dependent and was therefore used for calculations of tumor tracer uptake. SUVmean was used for measuring uptake in healthy organs. CT findings were evaluated by a radiologist. All tumor lesions measuring at least 10 mm on the baseline CT scan were noted for comparison with PET. Treatment response was assessed according to RECIST 1.1.
Biomarker Analysis
Serum samples were collected before every tracer injection—at days −4, 11, and 39—for analysis of circulating VEGF-A levels, with day 0 being the day of the baseline PET scan and the start of everolimus treatment. The blood was centrifuged, and platelet-poor plasma samples were stored at −80°C until analysis. VEGF-A was analyzed using an enzyme-linked immunosorbent assay (R&D Systems). Plasma VEGF-A was compared with tumor tracer uptake.
Statistical Assessments
We estimated that to predict with 80% power (2-sided α = 0.05) that there is a true difference in SUV (≥1.25 SD) between the baseline scan and the scan after 2 or 6 wk, the required number of patients would be 11. The change in 89Zr-bevacizumab tumor lesion uptake between the baseline scan and the scan after 2 or 6 wk of treatment was calculated and presented as a percentage (mean with 95% confidence interval). The Wilcoxon paired rank test was used to assess the significance of the change in tumor and healthy organ tracer uptake and plasma VEGF-A. Correlations were analyzed using the Spearman rank test.
Analyses were performed with SPSS, version 22 (IBM).
RESULTS
Patients
Between December 2009 and October 2014, 13 patients were included. The median interval between the stopping of tyrosine kinase inhibitor and the starting of everolimus was 10.57 wk, with a mean of 16.8 wk (range, 2–44.3 wk). Ten patients completed the study; 2 patients stopped everolimus between 2 and 6 wk because of adverse events, and one patient had clinically progressive disease before the third PET scan. The median time to progression was 5.8 mo (range, 1.9–24.9 mo). The patient characteristics are presented in Table 1.
Patient Demographics and Clinical Characteristics
89Zr-Bevacizumab PET
89Zr-Bevacizumab Uptake in Tumor Lesions
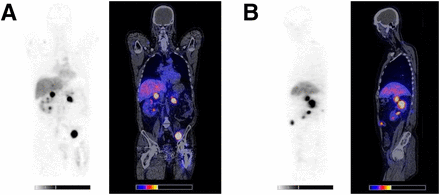
89Zr-bevacizumab PET images were of high quality and visualized tumor lesions clearly (Fig. 1). Of 147 tumor lesions 10 mm or larger on CT, 103 were detected as hot spots on the PET scan (71%). A total of 94 tumor lesions fulfilled the criteria for reliable quantification and were used for calculations (Fig. 2).
Baseline 89Zr-bevacizumab PET images of mRCC patient. (A) Coronal 2-dimensional image through right adrenal gland metastasis, showing additionally 1 local recurrence in left kidney tumor, 1 kidney metastasis, and 1 soft-tissue metastasis near left iliac bone. (B) Sagittal 2-dimensional image through right adrenal gland metastasis, showing additionally 3 kidney metastases and 1 soft-tissue metastasis in abdominal wall.
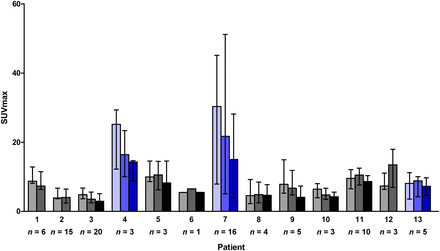
Median tumor tracer uptake with interquartile range per patient at baseline (light shading), after 2 wk of everolimus (medium shading), and after 6 wk of treatment (dark shading). Patients in blue stayed on treatment > 12 mo. n = number of quantified tumor lesions per patient.
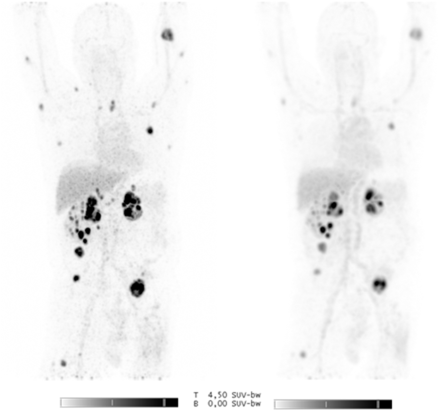
The median SUVmax at baseline was 7.3 (range, 1.6–59.5). All 13 patients underwent the second PET scan after 2 wk of treatment. The median SUVmax was 6.3 (range, 1.7–62.3), corresponding to a mean change of −9.1% (95% confidence interval, −3.4% to −14.9%, P < 0.0001). On the third PET scan, performed on 10 patients, 70 evaluable lesions were apparent, with a median SUVmax of 5.4 (range, 1.1–49.4). This corresponds to a mean change of −23.4% (95% confidence interval, −16.5% to −30.2%, P < 0.0001) compared with baseline (Figs. 3–5).
(Left) Maximum-intensity projection before treatment showing multiple local recurrences in left kidney tumor and metastases in right kidney, right adrenal gland, and soft-tissue. (Right) Maximum-intensity projection after 6 wk of treatment showing heterogeneous decrease in uptake.
Tumor tracer uptake before and during treatment. (A) Median uptake and interquartile range for all lesions. (B) Mean tumor uptake per patient. *P < 0.0001.
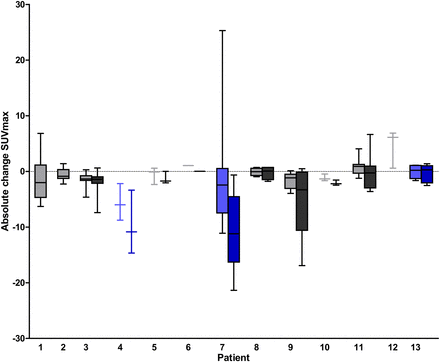
Absolute SUVmax change with median for all lesions per patient after 2 wk (light shading) and 6 wk (dark shading) of everolimus treatment. Patients in blue stayed on treatment > 12 mo.
89Zr-Bevacizumab Uptake in Healthy Organs
89Zr-bevacizumab uptake in healthy organs at baseline was comparable to previous studies (Supplemental Fig. 2) (12,15).
89Zr-Bevacizumab PET and Treatment Outcome
All 10 patients still on everolimus after 3 mo had stable disease according to RECIST 1.1 at the first response evaluation. The single patient with clinical progressive disease within 3 mo had a baseline median tumor SUVmax of 8.7 (range, 6.8–14.9) and a mean decrease of 12.2% in tumor SUVmax after 2 wk of treatment, which resembles the pattern seen in patients without early progression. Exploratory analysis showed a correlation between baseline mean tumor SUVmax and time on treatment (r = 0.63, P = 0.02, Supplemental Fig. 3). The mean tumor SUVmax at baseline of patients who were on treatment longer than 12 mo was higher than the mean tumor SUVmax of patients who were on treatment less than 12 mo (median mean tumor SUVmax, 22.2 vs. 7.2) (P = 0.09).
There was no correlation between change in SUVmax after 2 and 6 wk of treatment and time on treatment.
Plasma VEGF-A
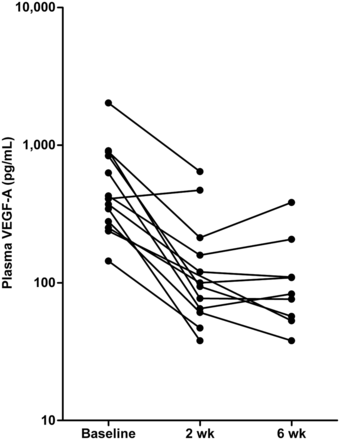
The median baseline plasma VEGF-A was 408 pg/mL (n = 13; range, 144–2,013 pg/mL). At day 11, the median plasma VEGF-A was 97 pg/mL (n = 12; range, <38–644 pg/mL), representing a mean decrease of 68.7% (P = 0.001). After 6 wk, 10 patients were ongoing, and 9 samples were available. The median VEGF-A was 83 pg/mL (range, <38–384 pg/mL; decrease, 74.6%; P = 0.004) (Fig. 6). Only one patient showed an increase in plasma VEGF-A level after 2 wk of treatment; this patient had early clinical progression. At baseline, there was no correlation between plasma VEGF-A and tumor SUVmax. There was also no correlation between change in tumor tracer uptake and change in plasma VEGF-A after 2 or 6 wk.
Plasma VEGF-A concentrations. Each line represents one patient.
DISCUSSION
In this study on mRCC patients, we found a decrease in 89Zr-bevacizumab tumor uptake after 2 wk of everolimus treatment and a further decrease after 6 wk. Because all patients had stable disease on CT at 3 mo, we could not assess the use of 89Zr-bevacizumab PET for early identification of progressive disease. Substantial heterogeneity in tumor tracer uptake both within and between patients was seen at baseline, but also the change in tumor tracer uptake during treatment varied considerably.
Predictive biomarkers that can differentiate between patients likely to benefit from mTOR inhibitors and those unlikely to benefit are needed to achieve patient-tailored therapy. Several potential markers have been identified, such as functional mutations in the mTOR pathway genes, baseline serum lactate dehydrogenase, and off-target effects resulting in specific side effects (16–19). However, these markers still have to be validated, and their potential clinical usefulness has to be established.
18F-FDG PET has been investigated as a predictive biomarker for everolimus treatment in 50 mRCC patients. There was no correlation between baseline SUVmax and change in tumor size, but baseline SUVmax showed a significant negative correlation with OS and PFS. A modest correlation between change in SUVmax and change in tumor size was found, but change in SUVmax did not correlate with OS or PFS. Altogether, the investigators did not recommend any additional studies (20).
We developed 89Zr-bevacizumab PET imaging to noninvasively study tumor VEGF-A concentration. Previously, we performed serial 89Zr-bevacizumab PET scans (at baseline and after 2 and 12 wk) on patients who started everolimus treatment for neuroendocrine tumors (15). In that study, tumor lesions were visualized in 10 of 14 patients, with a median SUVmax of 5.8 (range, 1.7–15.1). Tumor SUVmax decreased by 7% after 2 wk of treatment and by 35% after 12 wk, which mirrors the results of the current study on mRCC patients. The highest tumor SUVmax in neuroendocrine tumor patients, however, was 15.1, whereas in the current study on mRCC patients the highest was 59.5. Furthermore, only 19% of all lesions 10 mm or larger were visualized in the 10 neuroendocrine tumor patients with a positive PET scan, whereas in the present study we visualized 71% of mRCC lesions 10 mm or larger. These differences are likely explained by tumor biology. Sporadic VHL mutations are a unique characteristic of RCC and result in high VEGF-A production by tumor cells.
In the current study we did not perform biopsies. However, a good correlation between tumor uptake of radioactive labeled bevacizumab and VEGF-A in tumor tissue was shown earlier (13,21,22). We therefore assume that the decrease in tumor tracer uptake represents a reduction in VEGF-A production by the tumor cells as a result of everolimus treatment. Another potential explanation may be antiangiogenic effects of everolimus, such as reduced tumor perfusion or reduced vascular permeability (23). However, dynamic contrast-enhanced MRI using Ktrans measurement in patients with advanced cancer showed no decrease in tumor perfusion on everolimus treatment (24). Whether mainly tumor VEGF-A concentration or also antiangiogenic effects of everolimus are visualized with serial 89Zr-bevacizumab PET remains to be determined.
Patients who participated in the current study received prior treatment with a tyrosine kinase inhibitor. In a previous study, we demonstrated that sunitinib results in a heterogeneous change in tumor 89Zr-bevacizumab uptake, with an overall slight decrease and a rebound phenomenon after 2 stop weeks (12). The median interval between the stopping of tyrosine kinase inhibitor and the starting of everolimus was 10.57 wk (range, 2–44.3 wk). For the 2 patients who had an interval of 2 wk between tyrosine kinase inhibitor and tracer injection, we cannot exclude the possibility that prior treatment influenced tumor tracer uptake. For the overall study results, however, any influence from previous treatment is likely limited.
Median plasma VEGF-A decreased after 2 wk of everolimus treatment. Overall, a small further decrease was measured after 6 wk, but in half the patients plasma VEGF-A increased between weeks 2 and 6.
The current study was conducted before publication of the CheckMate 025 and METEOR trials (11,25). Nivolumab, a programmed-death-1–targeting antibody that acts as an immune checkpoint inhibitor, prolonged OS compared with everolimus in previously treated patients with advanced RCC. Cabozantinib, a tyrosine kinase inhibitor of VEGF receptors, MET, and AXL, was also shown to be superior to everolimus in previously treated mRCC patients. These trials strongly enforce the need for predictive biomarkers that can identify patients who will likely benefit from everolimus to allow individualized treatment choices. In an exploratory analysis, we found that the baseline 89Zr-bevacizumab PET results correlated with time on treatment. Tumor tracer uptake was higher in patients who were treated longer. Therefore, 89Zr-bevacizumab PET might be useful for predicting treatment success.
Previously, we published results on serial 89Zr-bevacizumab PET imaging of mRCC patients who were treated with bevacizumab/interferon alfa or sunitinib. A similar heterogeneous tumor tracer uptake at baseline was found with a slightly lower detection rate of tumor lesions 10 mm or larger (56.7%). A correlation between baseline tumor tracer uptake and time to progression was shown in an exploratory analysis, with a high uptake corresponding to a longer time to progression (12). This observation, in combination with the current study, supports the option that 89Zr-bevacizumab PET has prognostic rather than predictive value. Interestingly, in nephrectomy samples from 137 patients with locally advanced or metastatic clear cell RCC, high VEGF expression determined by immunohistochemistry was associated with worse OS. Median OS was 206 mo in patients with low VEGF expression and 65 mo in patients with high VEGF expression (P < 0.001) (26). This observation raises the possibility that high tumor VEGF-A is an unfavorable prognostic factor but—in the metastatic setting, by providing a target for treatment—identifies patients that show prolonged benefit from direct and indirect VEGF pathway inhibition with angiogenesis and mTOR inhibitors.
Larger trials are needed to evaluate whether this molecular imaging tool can serve as a predictive biomarker to select patients who derive clinically relevant benefit from everolimus.
CONCLUSION
89Zr-bevacizumab tumor uptake decreases significantly during everolimus treatment in mRCC patients. We could not demonstrate whether 89Zr-bevacizumab PET can be used for early identification of progressive disease. Larger trials are warranted to determine whether 89Zr-bevacizumab PET can be used to select patients for everolimus treatment.
DISCLOSURE
This work was supported by Novartis and the Dutch Cancer Society (RUG 2012-5565). No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jan. 12, 2017.
- © 2017 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication September 1, 2016.
- Accepted for publication October 24, 2016.