Abstract
Molecular imaging uses noninvasive techniques to visualize various biologic pathways and physiologic characteristics of tumors and normal tissues. In relation to radiation therapy, PET with the tracer 18F-FDG offers a unique opportunity to refine the target volume delineation in patients with squamous cell carcinoma of the head and neck, in turn affecting dose distribution and, it is hoped, patient outcome. Even more so, in the framework of adaptive treatment and theragnostics, whereby dose distribution is adapted in space and time over the typical course of radiotherapy, molecular imaging with PET offers an elegant research avenue to further improve the therapeutic ratio. Such implementation could be of particular interest with tracers other than 18F-FDG, such as tracers of hypoxia and proliferation.
Molecular imaging, also referred to as biologic or functional imaging, uses noninvasive techniques to visualize various biologic pathways and physiologic characteristics of tumors and normal tissues. It refers mainly (but not only) to PET and functional MRI. In clinical oncology, molecular imaging offers the unique opportunity of allowing earlier diagnosis and staging of disease; contributes to the selection and delineation of optimal target volumes for radiotherapy and, to a lesser extent, surgery; assesses response early during treatment or after its completion; and helps in the early detection of recurrence (1,2).
It appears that more and more radiation oncologists are routinely using information provided by 18F-FDG PET to select or delineate target volumes in patients with squamous cell carcinoma of the head and neck (HNSCC). However, how do the figures for the specificity and sensitivity of 18F-FDG PET compare with those of anatomic imaging such as CT or MRI in the diagnosis of primary tumors and metastatic lymph nodes in the neck? How does 18F-FDG PET–based delineation of primary tumor gross target volume (GTV) compare with CT- or MRI-based GTV? How does the use of 18F-FDG PET–based volumes modify the dose distribution, allowing for normal tissue sparing, dose intensification, or dose painting? Are other tracers such as hypoxic or proliferation tracers of any use?
This review of state-of-the-art therapeutic management of patients with HNSCC will present all available data justifying the use of PET with 18F-FDG or other tracers in these patients. Only squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx, and neck metastasis of an occult primary, will be discussed.
18F-FDG PET FOR TARGET VOLUME SELECTION
The use of 18F-FDG PET in HNSCC has been proposed in many studies, and several meta-analyses have tried to assess the level of evidence for this technique in various clinical scenarios. Although published data do not indicate a clear role for 18F-FDG PET in HNSCC patients, technology is evolving quickly in this field and published data indicating merit are often from studies conducted with scanners that can no longer be considered the state of the art.
Indications addressed in published reports are related mostly to the diagnostic accuracy of PET in the detection of an occult primary tumor in patients with cervical lymph node metastases. These tumors might not have been identified on clinical examination and imaging, or they might only be suspected with other diagnostic modalities. In this clinical scenario, 18F-FDG PET is able to detect about one quarter of occult primaries (3), but its value over CT and MRI still needs to be confirmed. Besides the identification of the occult primary tumor, the added value of PET is in the detection of distant metastases—a use for which it has shown high sensitivity. Clearly, the discovery of metastatic lesions has a great impact on patient management (4).
Several studies have been published on the assessment of HNSCC primary tumor using PET, with reported sensitivities, specificities, and accuracies of over 90% (5). Nonetheless, PET has not been shown to have any advantage over CT and MRI and is therefore not recommended currently for routine diagnostic imaging of primary head and neck cancer. New studies using integrated state-of-the-art PET/CT scanners might change this indication.
The role of PET in the identification of lymph node metastases was the subject of a recent meta-analysis (6). Sensitivity and specificity were not significantly higher for 18F-FDG PET than for ultrasound, CT, or MRI. In patients with clinically negative neck nodes, PET sensitivity was as low as 50%, without any advantage over conventional imaging techniques, particularly when compared with ultrasound-guided fine-needle aspiration cytology.
Although 18F-FDG has a limited role for the primary tumor and node staging, it is considered appropriate for the evaluation of patients after radiation therapy or chemotherapy with or without a clinical or imaging suspicion of relapsing disease (7). In this setting, particular attention must be paid to inflammatory changes due to radiation therapy, which may easily lead to false-positive reports. It is beyond the scope of this review article to extensively discuss these indications and pitfalls.
18F-FDG PET FOR TARGET VOLUME DELINEATION AND DOSE PLANNING
Accurate evaluation of the primary tumor and its extensions remains challenging and difficult for the radiation oncologist. This issue is crucial because the GTV represents the volume that has the highest tumor cell density and should most accurately receive the prescribed dose. GTV delineation relies mainly on a comprehensive physical examination and the use of optimal imaging modalities but also on sound clinical judgment and in-depth knowledge of head and neck anatomy and the pathways for tumor spread. The imaging modalities currently available can offer anatomic (e.g., CT and MRI) or functional (e.g., PET) information, but all present limitations in spatial or contrast resolution and in sensitivity and specificity. These limitations may significantly affect image interpretation and subsequent tumor delineation.
In HNSCC, both GTVs and organs at risk are typically delineated on planning CT. CT presents numerous advantages, including its widespread availability, acceptable cost, high spatial resolution, and intrinsic information on the electronic density used for dose calculation. However, CT lacks contrast resolution for soft tissues and tumor extent and is sensitive to metallic artifacts, such as dental fillings, limiting its performance in assessing oropharyngeal and oral cavity tumors. The use of contrast-enhanced CT and the availability of delineation guidelines have been shown to improve consistency between observers (8), emphasizing the importance of image quality and guidelines for delineation criteria. In comparison to CT, MRI offers better soft-tissue contrast, which may improve the delineation of GTV near the base of the skull, such as in nasopharyngeal or paranasal sinus cancers and in tongue, base-of-tongue, and floor-of-mouth tumors. However, for pharyngolaryngeal tumors, several studies have failed to show any added value of MRI over CT in volumetrics and reproducibility, even when optimal MRI sequences with high spatial resolution were used (9).
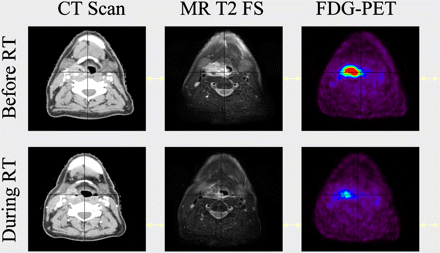
Besides these anatomic imaging modalities, the use of 18F-FDG PET, which provides unique information on cellular function, has become increasingly popular in radiotherapy planning. In pioneering work, Daisne et al. demonstrated that the introduction of 18F-FDG PET for the delineation of pharyngolaryngeal squamous cell carcinoma led to significantly smaller GTVs than those delineated on CT or MRI (10). A comparison with surgical laryngeal specimens showed that GTVs delineated from 18F-FDG PET were the closest to the pathologic GTV, whereas the use of CT or MRI led, on average, to an overestimation of the true tumor volume by 40% and 47%, respectively. These results highlight that the lack of specificity of these anatomic imaging modalities may result in inadequate target definition, with possible consequences on dose distribution. Although 18F-FDG PET seems a good candidate for radiotherapy planning, its optimal use is not a trivial task. The physics of PET, including poor statistics and poor spatial resolution, result in highly noisy and blurred images that may severely affect accurate determination of the volume and shape of tumors (Fig. 1).
Patient with stage T2 N0 M0 right hypopharyngeal HNSCC treated with concomitant chemotherapy and radiotherapy. Patient was imaged with intravenous contrast CT, MRI (fat-saturated [FS] T2-weighted sequence), and 18F-FDG PET before treatment and at end of week 3 (30 Gy). Primary tumor shrinkage was observed with all imaging modalities but was more dramatic with 18F-FDG PET.
It is beyond the scope of this article to extensively review all available methods for 18F-FDG PET volume segmentation (11). Visual approaches are highly subjective and affected by display windowing, and methods based on a fixed threshold of activity are far too simplistic, as the optimal threshold varies substantially from case to case. More sophisticated methods include adaptive threshold–based approaches such as signal-to-background ratio, which was developed by our group. Although validated, this method cannot easily be generalized, as it is dependent on camera and reconstruction algorithm and is not very accurate for images with a low signal-to-background ratio, as observed during treatment. The use of gradient-based segmentation represents another method, which was motivated mainly by the intrinsically low quality of PET images—that is, the high level of noise and blurred images. Such a method was made possible by the implementation of specific image restoration tools, that is, edge-preserving filters for noise removal and deconvolution algorithms for deblurring (12). This segmentation technique has proven to be more accurate and reliable than threshold-based methods, especially in segmenting images acquired during radiotherapy (13).
With these adequate segmentation tools at hand, 18F-FDG PET appears promising because it provides smaller, more accurate and reproducible GTVs than does CT or MRI (10). More important, refining the GTV delineation by means of 18F-FDG PET ultimately led to better dose distributions within planning target volumes and surrounding tissues (9,14). Indeed, the reduction of the primary tumor target volumes allowed by such an approach translated into significant decreases in the volumes receiving the highest dose using either 3-dimensional conformal radiotherapy or intensity-modulated radiotherapy with helical tomotherapy. A prospective multicentric phase II study has been performed to clinically validate this concept in HNSCC. Results should be available by mid-2011.
In view of the growing role of imaging in target definition for radiotherapy, one should keep in mind that all macroscopic modalities available will fail to depict subtle tumor extensions and, in particular, the mucosal extent of the disease. As shown by Daisne et al. (10), if CT, MRI, and 18F-FDG PET overestimate the GTV in most dimensions, all 3 imaging modalities fail to accurately assess the mucosal invasion visualized on the macroscopic specimen. In this context, a careful physical examination, including endoscopy and palpation, still remains the best method for appreciating the mucosal extent of HNSCC.
ADAPTIVE TREATMENT AND THERAGNOSTICS
Significant progress in imaging, dose calculation, and delivery has recently opened avenues for new treatment opportunities such as adaptive radiation therapy and dose painting. Adaptive radiation therapy consists of reassessing tumor volume after a given dose of radiation has been delivered and then boosting the residual imaged tumor. Preliminary studies have already demonstrated the feasibility and usefulness of such approaches in pharyngolaryngeal squamous cell carcinoma and showed significant tumor shrinkage using both anatomic and functional imaging (14). Such approaches, consisting of boosting (dose > 2 Gy per fraction) the shrinking tumor volume, are safer than reducing the irradiated volume, which could lead to missed invasive cells at the edge (15). The selection of small volumes for dose escalation strategies is mandatory to prevent unacceptable damage to critical structures embedded within the boost volumes, and even more so when a high dose per fraction is prescribed, such as in simultaneous integrated boost intensity-modulated radiotherapy.
Another appealing approach is the integration of biologic information from molecular imaging modalities with the purpose of targeting radiation-resistant regions inside the tumor, such as high clonogen density, proliferation, or hypoxia, hypothesizing that the regionally variable radiosensitivity may require a heterogeneous dose distribution to achieve optimal tumor control. In all studies on target volume definition conducted so far, it has been assumed that even when defined with regard to specific biologic pathways, GTVs were homogeneous and did not vary during the course of radiation treatment. Hence, a radiation dose homogeneously distributed in space and time is delivered. This assumption is likely an oversimplification of the biologic reality, as tumors are known to be heterogeneous with respect to pathways of importance for radiation response, and at least some of them are known to progressively shrink during treatment.
Bentzen proposed the term theragnostic to describe the use of molecular imaging to prescribe the distribution of radiation dose in 4 dimensions, that is, the 3 spatial dimensions plus time (16). This is undoubtedly a challenging research topic that could potentially revolutionize the process of radiotherapy planning and delivery. However, before such a dream can become a reality, several issues need to be resolved.
One critical issue is the ability to accurately visualize in space and time the exact location of those clonogenic cells expressing a phenotype that may require the delivery of an extra radiation dose. This issue addresses not only the availability of specific tracers for the various biologic pathways of interest but also the spatial resolution and correctness of the available imaging modalities. In a recent study performed on mouse tumor models, Christian et al. found spatial discrepancies between the PET images and the underlying microscopic reality represented by autoradiography images (17). Such differences, attributed to the finite resolution of PET, were important when small and highly active regions of the tumors were considered. Another critical issue for theragnostics is the need to establish correspondence between a PET signal intensity (or a PET image segmentation) and a prescribed dose, thus evolving the concept of dose painting into dose painting by numbers. As another challenge, tools are needed to register in space and time the various images and the dose distribution acquired throughout the therapy. To this end, nonrigid registration techniques will be required, as tumor or normal-tissue shrinkage is expected during the course of radiotherapy treatment (18). Lastly, from a biologic point of view, the challenge is to relate a change in tracer uptake to a change in the underlying biology, thus requiring a comprehensive biologic validation of the concept of dose painting or dose painting by numbers in experimental models. In summary, although much still needs to be done, it is likely that over the next 5–10 years we will witness some of our dreams coming into reality.
TRACERS OTHER THAN 18F-FDG FOR TARGET VOLUME DELINEATION
The identification of hypoxic tumor volume that can be prescribed higher doses of radiation has been the subject of numerous studies. These studies are aimed at identifying the best radiopharmaceutical for this particular clinical setting. 60Cu- and 64Cu-labeled methylthiosemicarbazone, 18F-fluoromisonidazole, and 18F-fluoroazomicyn arabinoside are, among others, the radiopharmaceuticals proposed in these reports (19). Most studies have attempted to identify the hypoxic subvolume within the GTV, to allow the use of higher doses in the hypoxic cells (20–23). Although 18F-fluoromisonidazole has known limitations in contrast resolution and uptake time, studies with this tracer have demonstrated the possibility of hypoxic volume identification. Other tracers, such as 18F-fluoroazomicyn arabinoside and methylthiosemicarbazone, are probably going to be applied in large series of patients. It is hoped that future large clinical trials will demonstrate the usefulness of these radiopharmaceuticals and improve the selectivity of radiation treatments. It is reasonable to forecast that these tracers will not be used in diagnosis—where 18F-FDG is going to remain the major player—but will find a paramount role in the biologic characterization of tumors before, during, and after therapy.
Another clinical need for new radiopharmaceuticals derives from the high number of false-positive findings due to inflammatory changes that are reported in 18F-FDG studies. To increase low 18F-FDG specificity, the use of 18F-fluorothymidine has been proposed. This tracer probes increased DNA synthesis and is supposed to be more influenced by inflammatory processes in tissues and lymph nodes. 18F-fluorothymidine PET showed promising results in radiation therapy applications; therefore, more studies are expected in the coming years (24). Other compounds—such as 18F-fluoroethyl-tyrosine and 11C-methionine—able to visualize cellular amino acid uptake for protein synthesis have been proposed as well to increase the specificity of PET (24). Although many other PET compounds are under evaluation, 18F-fluorothymidine seems to be the most promising because of its high specificity. In these settings, 18F-FDG will probably remain the first radiopharmaceutical to be used to study tumors for which it has high sensitivity. More specific tracers, such as 18F-fluorothymidine, will be used as a way to further characterize suspected lesions when false-positive findings cannot be excluded.
- © 2011 by Society of Nuclear Medicine
REFERENCES
- Received for publication June 28, 2010.
- Accepted for publication September 30, 2010.